Reference no: EM13884282
In the manufacture of methyl ethyl ketone from butanol, the product is separated from unreacted butanol by distillation. The feed to the column consists of a mixture of 0.90 mol fraction MEK, 0.10 mol fraction 2-butanol, with a trace of trichloroethane.
The feed rate to the column is 20 kmol/h and the feed temperature 35 °C. The specifications required are: top product 0.99 mol fraction MEK; bottom product 0.99 mol fraction butanol.
Design a column for this separation. The column will operate at essentially atmospheric pressure. Use a reflux ratio 1.5 times the minimum.
a. Determine the minimum reflux ratio.
b. Determine the number of theoretical stages.
c. Estimate the stage efficiency.
d. Determine the number of actual stages needed.
e. Design a suitable sieve plate for conditions below the feed point
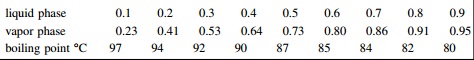
Equilibrium data for the system MEK -2-butanol, mol fractions MEK:
|
Explain why step uniformity is so important for stairways
: Explain why step uniformity is so important for stairways.
|
|
Does the study test a stated hypothesis
: What is the study question and is it relevant ,Does the study add anything new and What type of research question is being asked?
|
|
Procedures were performed to address presentation
: The following audit procedures were performed to address presentation and disclosure related audit objectives related to notes payable.
|
|
Determine dells tax rate by using the income tax rate
: Determine the annual depreciation by assuming Dell depreciates these assets by the straight-line method over a 5-year life and determine Dell's tax rate by using the income tax rate in 2012.
|
|
Operate at essentially atmospheric pressure
: Design a column for this separation. The column will operate at essentially atmospheric pressure. Use a reflux ratio 1.5 times the minimum.
|
|
What is their multi-factor productivity currently
: 1Charles Bakery in Idaho, makes 1,500 loaves every month. They estimate the demand to be 25% more than their current sales. Their current single factor productivity is 2.344 loaves per labor-hour.
|
|
Advantages of using electronic health records
: advantages of using electronic health records.
|
|
Audit interest expense and interest payableaccounts
: Your client, Red Horse, Inc., prepared the following schedule for long term debt for the audit of financial statements for the year ended December 31, 2013:
|
|
Size distribution of the particles entering the filters
: For a gas flow rate of 100,000 m3 /h, at the reactor conditions, determine how many cyclones operating in parallel are needed and design a suitable cyclone. Estimate the size distribution of the particles entering the filters.
|