What is Ionic bonds ?
Ionic Bonds : Ionic bonds hold atoms together in crystals. They form when oppositely charged atoms, or ions, join (opposite charges attract) to equalize the overall charges, resulting in the formation of an ionic compound.
Ions are formed when an atom or group of atoms gains or loses electrons. Normally, the number of an atom's negatively charged particles equals the number of positively charged particles, and the sum of the charges for the atom is neutral. When an atom, usually a nonmetal, attracts an extra electron or electrons in order to become more stable, it becomes an ion. Positively charged ions are called cations, and negatively charged ions are called anions. Atoms that lose negative electrons become more positive, and those that gain negative electrons become more negative.
Nonmetals typically have shells that contain 5, 6, or 7 electrons in their outer shells, and therefore require one, two, or three electrons to become more stable by filling the shell. Nonmetals tend to gain electrons. Metals, having one, two or three electrons in their outer shells usually tend to readily lose one or more electrons to become more stable themselves.
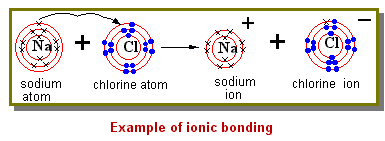
In this example, a sodium atom loses an electron to become a positively charged ion (cation), while a chlorine atom gains an electron to become a negatively charged ion (anion). The two oppositely charged ions then join to form a neutral compount, NaCl, sodium chloride, commonly known as table salt.