Q. Illustrate Oxidation States of f-block?
The addition of the first three ionisation energies of the lanthanides is comparatively low, so the elements are highly electropositive. They readily form M3+. For the actinium, lanthanides and trans-americium (Cm to Lr) elements the tripositive oxidation state is the most stable in every case. It is known that in forming tripositive lanthanide or actinide ions, the ns2 (n = 6 or 7) electrons are lost along with the (n - l)dl electron. In the absence of (n - 1)d' electron, one of the electrons present in the (n - 2)f orbitals is lost.
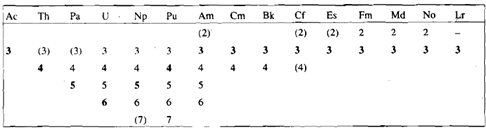
Besides the +3 state, some of the actinides and lanthanides show other oxidation states also. In these cases there is some evidence that ions with f0 (eg..La3, Ce4+, Ac3+, Th3+, Pa5+, U6+)f7 (e, g Eu2+, Gd3+, Tb4+,Cm3+, Bk4+) and f14 ( e, g Yb2+, Lu3+ configurations exhibit greater stability. However, pr4+ (4fl) Nd4+, (4f), Sm2+ (4f6), Tm2+ (4f13) etc. with non--, non-f7 and non f 14 electronic configurations also exist. This reminds us that there can be other factors also such. as ionisation energies and sublimation energies of the metals and lattice energies, etc., which are responsible for the stability of these oxidation states. The defined oxidations states of actinium and the actinides are given in Table in which numbers in bold indicate the most stable oxidation state in aqueous solution. You will see from the Table that nearly all the actinides exhibit at least two stable oxidation states and oxidation states higher than +3 are easily accessible in the early actinides. For protactinium, thorium and uranium the highest accessible oxidation state is the most stable one also in aqueous solution. This can be because Sf orbitals extend further from the nucleus than the 4f orbitals and Sf electrons are more effectively shielded from the nuclear charge than are the 4f electrons of the corresponding lanthanides. As the Sf electrons are less firmly held, they are all available for bonding in the early actinides. Though, as the later actinides are approached, the build-up of nuclear charge causes contraction of the Sf orbitals so that the metal-ligand overlap decreases and the +3 state become predominant. Interestingly, the +2 state which achievable in case of mendelevium and nobelium is more stable than EU2+.