Reference no: EM13294518
(1) Indicate whether each of the following molecule pairs are (a) identical, (b) enantiomers, or (c) diasteriomers.
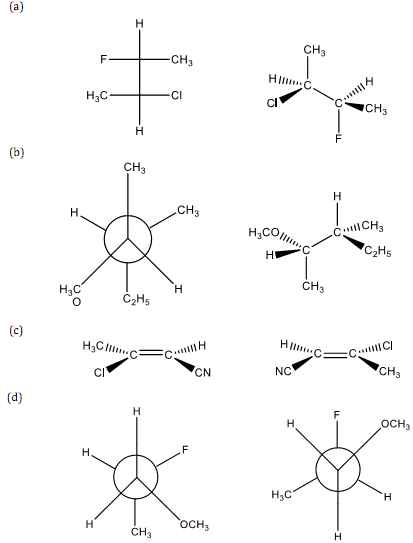
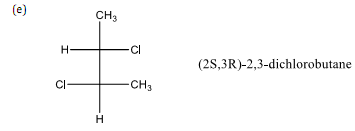
(2) Draw the 3-dimensional representation for each of the following.
(a) (2S,3R)-2-methoxy-3-chlorohexane
(b) (R)-1,1,2-trimethylcyclohexane
(3) Write the complete IUPAC name for the following compounds.
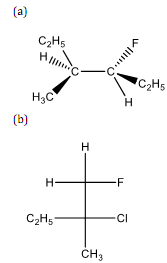
(4) Designate True (T) or False (F) for each of the following statements.
(a) A sample of an optically active substance that consists of a single enantiomer is said to have an enantiomeric excess of 100%.
(b) The (R) enantiomer of a chiral compound always rotates the plane of polarized light to the right.
(c) The meso form of a molecule can be either dextrorotatory or levorotatory.
(d) 3-Methylcyclohexene is chiral.
(e) trans-1,3-dichlorocyclohexane is chiral but the cis-isomer is not.
(f) The difference in free energy between the reactants and the product is defined as the free energy of activation.
(g) The first step in the SN1 reaction of tert-butyl chloride is the homolytic cleavage of the carbon-chlorine bond forming the tert-butyl carbocation and a chloride ion.
(h) Carbon-hydrogen and carbon-carbon bonds located beta to a carbocationic center stabilize the cationic species by hyperconjugative electron delocalization.
(i) The Hammond-Leffler postulate states that the structure of a transition state resembles the stable species that is nearest it in free energy.
(j) Nucleophilicities are related to equilibria while basicities are related to rates of reaction.
(k) Dimethyl formamide and dimethylsulfoxide are polar protic solvents.
5. Complete each of the following reactions. Show the stereochemistry of the product when appropriate.
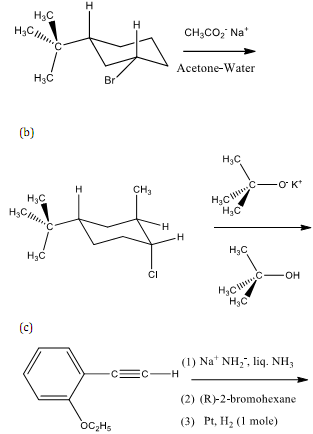
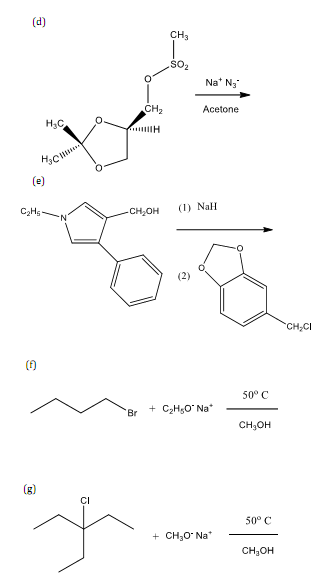
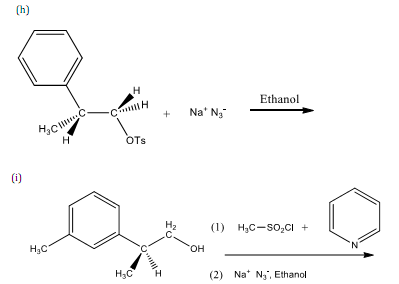
6. Answer each of the following questions.
(a) For each of the following circle which reaction will occur faster.
(1) 1-Bromobutane or 1-Iodobutane with sodium azide in dimethyl sulfoxide.
(2) 1-Bromo-2-methylbutane or 1-bromopentane with sodium iodide in acetone.
(3) 1-chlorohexane or cyclohexyl chloride with sodium azide in aqueous ethanol.
(4) Solvolysis of 1-bromo-2,2-dimethylpropane or tert-butyl bromide in ethanol.
(5) Solvolysis of isobutyl bromide or sec-butyl bromide in aqueous formic acid.
(b) Give the mechanistic symbols (SN1, SN2, E1, E2) that are most consistent with each of the following statements.
(1) Methyl tosylate reacts with potassium iodide in acetone only by this mechanism.
(2) Ethyl bromide, 1-bromopropane, 1-bromobutane, and 1-bromopentane react with sodium ethoxide in ethanol mainly by this mechanism.
(3) The substitution product obtained by acetolysis of tert-butyl bromide reacts mainly by this mechanism.
(4) The elimination product obtained by methanolysis of tert-butyl bromide reacts mainly by this mechanism.
(c) Arrange the following carbocations in the order of increasing stability.
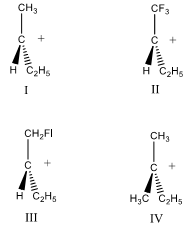
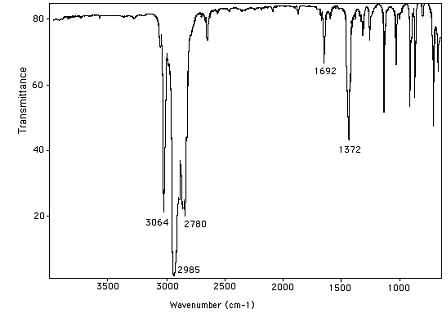
7. A molecule has the molecular formula C6H10. Analyzing the following infrared spectrum and suggest a reasonable structure for this molecule.
8. Which of the following molecules is consistent with the accompanying infrared spectrum?
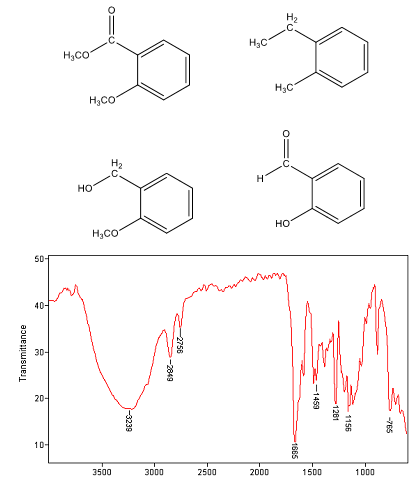
|
What is the monthly payment that can be made
: The fund will disburse monthly for 12 years, and the desired cash balance at the end of 12 years is $1,000,000. What is the monthly payment that can be made from this fund?
|
|
Define how many grams of nitrogen monoxide are present
: When 37.2 L ammonia and 42.0 L oxygen gas at STP burn, nitrogen monoxide and water are produced. After the products return to STP, how many grams of nitrogen monoxide are present
|
|
Bicycle frames using two fiberglass materials
: Applied Technology, Inc. (ATI) produces bicycle frames using two fiberglass materials that improve the strength-to-weight ratio of the frames. The cost of the standard grade material is $7.50 per yard and the cost of the professional grade mat..
|
|
Does the sequence converge in the mean square sense
: Let Un be a sequence of iid zero-mean,unit-variance Gaussian random variables. A "low pass filter" takes the sequence Un and produces the sequence Xn =1/2 ( Un +Un-1) a.) Does this sequence converge in the mean square sense
|
|
Write the complete iupac name
: Designate True (T) or False (F) for each of the following statements and a sample of an optically active substance that consists of a single enantiomer is said to have an enantiomeric excess of 100%.
|
|
Define the atomic weight of mn if the atomic weights
: What is the atomic weight of Mn if the atomic weights for Ag and Br are 107.868 and 79.904 respectively
|
|
What is the monthly payment that can be made from this fund
: The fund will disburse monthly for 12 years, and the desired cash balance at the end of 12 years is $1,000,000. What is the monthly payment that can be made from this fund?
|
|
Expanded growth in a certain portion of the city
: 2) Due to expanded growth in a certain portion of the city, a new waste truck capacity is needed. An additional truck can be purchased now to replace the presently owned assets. The city uses a 5% interest rate for project evaluation. It is eco..
|
|
Which would expect to have a bigger effect on cmos circuit
: Which would you expect to have a bigger effect on the power consumption of a CMOS circuit, a 5% increase in power-supply voltage or a 5% increase in internal and load capacitance
|