Reference no: EM131053894
Question 1
a) Choose the correct answers from the words given below
(HCl, Ag(NH3)2Cl, H2SO4, V2O5, N2, O2)
i) A salt obtained on reaction of a metallic chloride with Ammonia.
ii) The gas liberated when Ammonia is passed over heated PbO.
iii) The acid formed when Conc. HNO3 reacts with Sulphur.
iv) The catalyst used in the preparation of Sulphur trioxide from SO2 in contact process.
v) A gas which undergoes ionization completely when dissolved in water.
b) Match the following. Example- vi-F
Part-A
i) Inert electrode
ii) The ions which impart acidic nature to an acid
iii) Emulsifier
iv) Aqua regia
v) A non-volatile acid
vi) A basic gas
Part-B
A) Hydronium ions
B) Liquor ammonia
C) Conc. HNO3 + Conc. HCl
D) Iron
E) Conc.HCl
F) Ammonia
G) Conc. H2SO4
c) Select the correct answers from the words given below:
i) Ni - 2e- à Ni2+ is _______________ reaction.
A) an oxidation B) a reduction C) Redox D) synthesis
ii) An active electrode
A) Iron B) Platinum C) Carbon D) Copper
iii) The electrolyte used in the electroplating of an article with Silver:
A) AgNO3 B) Na[Ag(CN)2] C) AgCl2 D) KNO3
iv) Which of the following cation is reduced most readily:
A) Mg2+ B) K1+ C) Al3+ D) Cu2+
v) Sodium hydroxide solution is a __________
A) strong electrolyte B) weak electrolyte C) poor electrolyte D) non electrolyte
vi) Which of the following compounds liberates reddish brown vapours at anode during its electrolysis?
A)aq. NiSO4 B) aq.CuSO4 C) PbBr2 fused D) aq. PbBr2
vii) Hydrogen chloride gas is dried using __________.
A) Conc. H2SO4 B) Conc.HNO3 C) CaO D) CaCl2
viii) The suitable pressure in Haber's process to prepare Ammonia from Nitrogen and Hydrogen is:
A) 20atm B) 200-900atm C) 1-2atm D) 10-100atm
ix) The oxidized product formed when Sulphur is treated with Conc.H2SO4
A) SO2 B) S C) SO3 D) H2S
x) The salt formed when dil.H2SO4 reacts with insufficient Sodium hydroxide:
A) Na2SO4 B) NaHSO4 C) MgCl2 D) NaCl
d) Study the picture given and match A,B,C,D and E with the options given the brackets.
(Copper sulphate, Iron(II) sulphate, Zinc sulphate, Iron(III) chloride, Lead nitrate)
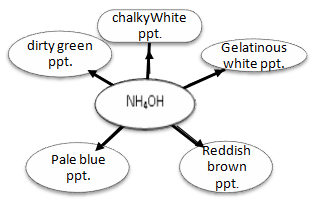
e) Fill in the blanks:-
i) Non-polar covalent compounds are _________ (non/good) electrolytes.
ii) Ammonia reduces to Chlorine to ____________( Hydrogen chloride/Sodium chloride)
iii) ____________ (Platinum/Iron) is used as a catalyst in the manufacture of Nitric acid by Ostwald's process.
iv) HCl gas is ____________ (lighter/heavier) than air.
v) H2SO4 is a ____________ (deliquescent/hygroscopic) liquid.
f) Correct the following sentences.
i) In an electrolysis anions migrate to cathode and cations migrate to anode.
ii) Phenolphthalein solution changes its colour from Orange to pink on passage of Hydrogen chloride gas through it.
iii) Ammonium hydroxide is also known as liquid ammonia.
iv) Conc. Nitric acid on thermal decomposition gives Nitrous oxide.
v) Conc. H2SO4 is a reducing agent.