Reference no: EM1342046
Question 1: Two well of radius 18 inches are constructed in a confined aquifer 150 ft thick with hydraulic conductivity K = 170 gpd/ft2 . When one well pumps at a rate of 225 gallons per minute (gpm), the steady-state piezometric head at the well perimeter is lowered 18 ft relative to the non-pumping level. When the pumping rate is doubled, the piezometric head drops an additional 12 ft. In both these tests, the second well is not operating. What is the drawdown at the perimeter of the well pumping 225 gpm when both wells are operating?
Question 2: Groundwater may contain considerable concentrations of reduced inorganic chemicals, particularly Mn2+ and Fe2+. When groundwater is contacted with chlorine for disinfection these metal ions are almost completely oxidized to Mn4+ and Fe3+, which form the insoluble hydroxides MnO2(s) and Fe(OH)3(s), respectively. For a groundwater containing 6 x 10-4 M Fe2+, how much chlorine gas (Cl2) is required to oxidize all the iron, if the Cl2 is converted to chloride ion, Cl-? How much solid sludge (in mg/L) is formed in the process?
Question 3: What is the alkalinity, in meq/L, of a solution at 20oC and in equilibrium with the atmosphere, if the solution pH is 9.5?
Question 4 : (Note: This is a more challenging question than I would ask on the exam, but it does contain key elements of solubility equilibrium that I hope you can understand. If you understand the steps used in the solution approach to this question, I think you have a thorough grasp of acid/base and solid/solution chemistry.) Phosphorus and nitrogen are sometimes recovered from digestor fluids by precipitating the mineral struvite, with a formula of MgNH4PO4. The reaction for dissolution of this mineral, and the corresponding value of Ksp are as follows:
MgNH4PO4(s) ↔ Mg2+ + NH4 + + PO4 3- Ksp = 10-13.26
If a digestor solution contains 10-2.5 M TOTMg, 10-2.8 M TOTNH4, and 10-3.0 M TOTPO4, and the solution pH is held steady at 9.8, how much struvite is likely to form? Note that NH4+ and PO43- are both participants in acid/base reactions; at pH 9.8, the fraction of the total acid/base groups represented by these species are:
[NH4+]/TOT NH4 = 0.218
[PO43-]/TOT PO4 = 2.65 X 10-3
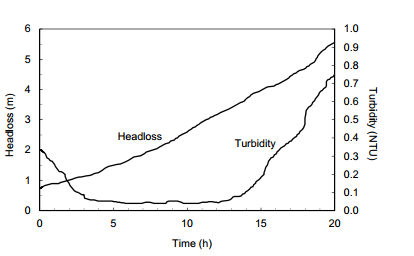
Question 5: The head loss and effluent turbidity during a typical filter run at a drinking water treatment plant are shown in the following diagram. The maximum head loss at which the filter can be operated efficiently is 2.5 m, and the turbidity criterion is 0.2 NTU. Approximately how long should the operator run the filter before backwashing?
Question 6: A river into which a city discharges its treated wastewater flows downstream for one day before it empties into a lake with an average hydraulic residence time of two weeks. The river can be treated as a PFR, and the lake is well-mixed. The flow in the river (including the wastewater) is 250 L/s, and the BOD utilization rate constant kd is 0.2/d in both the river and lake. The water entering the lake has an ultimate BOD of 24 mg/L. What is the rate of oxygen consumption in the lake?