Reference no: EM131637811
Chemical Engineering Homework-
Q1. A liquid mixture is 23 mole % benzene, 34 mole % acetic acid, and 43 mole % diethyl ether. Find the following.
a. Mass fractions of each component.
b. Average molecular weight of the mixture.
c. The mass (in grams) of a mixture if it contains 83 mol benzene.
Q2. A gas feed stream containing 4.03 mole % methane and balance O2 is mixed with a pure stream of O2. The final stream composition is 2.05 mole % methane. Determine the amount of O2 needed to dilute the feed stream.
Hint: Assuming a basis may be helpful.
Q3. Use the chemical engineering equipment encyclopedia to describe the following units in a way your parents or grandparents can understand.
a. Distillation column
b. Absorber
c. Crystallizer
d. Some other separations equipment of your choice.
e. Some other separations equipment of your choice.
Q4. Consider the following flow diagram. The process is designed to separate two components (A and B) from each other to produce various product and waste streams. All know data is shown. Assume that every stream has A and B. Product specifications require that the flow rate of the intermediate stream 1 be 50% of the feed stream flow rate. Determine the unknown flow rates and compositions.
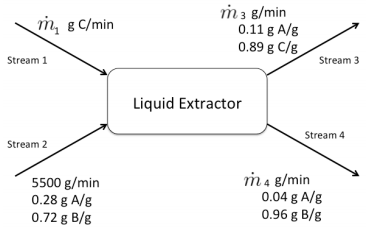
Q5. Liquid extraction is the removal of one liquid (A) from another liquid (B) through the use of a third liquid (C). Usually liquid B is immiscible in liquid C, while liquid A is miscible in liquid C. A solution of A/B is mixed with C, and the product streams are A/C and A/B solutions. In this example A is butanoic acid, B is water, and C is benzene. Below is a flowchart for such a process.
a. How many independent material balance equations can be written for this process?
b. Calculate m·1, m·3, and m·4 using the below information and solving the appropriate equations in the right order.
c. Show that the inlet flow rates of A, B, and C equal the outlet flow rates of A, B, and C.
d. One option to remove the butanoic from benzene is through distillation. Using the information so far, draw a two-unit process that may recover nearly pure butanoic acid.
Q6. An air stream contains 3 mol % water and flows into a column with calcium chloride pellets. The pellets absorb 95% of the inlet water and nothing else. The pellet weight before the air flow is 5.1 kg and after 10 hours the weight was 7.3 kg.
a. Determine the flow rate (molar) of the feed gas air.
b. Determine the water mole fraction of the outlet air gas.
c. The mole fraction of water in the outlet stream after 20 hours is the value calculated in part b, but then suddenly jumps to a large value. Give possible explanations for this? If the process is allowed to run a very long time, what water mole fraction do you expect the outlet stream to have?
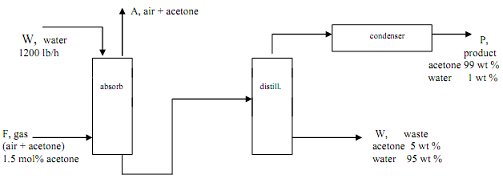
Q7. The process shown below removes a pollutant, acetone, from a gas stream before discharge to the atmosphere. Acetone is absorbed into the water, then purified in the distillation step. This not only cleans the air stream but provides revenue. EPA limits on the discharge require the air stream to have less than 0.005 weight fraction acetone.
a. Calculate all unknown flows and determine if the EPA regulation will be met.
b. If not met, what should be done?
Assume that 50% of the acetone fed to the process is recovered as product and that the concentration of acetone in the feed to the distillation unit is 0.5 wt fraction.
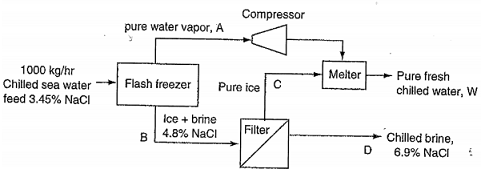
8. The figure below shows a schematic for making fresh water from sea water by freezing. The pre-chilled sea water is sprayed into a vacuum at a low pressure. The cooling required to freeze some of the feed sea water comes from evaporation of a fraction of the water entering the chamber. The concentration of the brine stream B, is 4.8% salt. The pure salt-free water vapor is compressed and fed to a melter at higher pressure where the heat of condensation of the vapor is removed through the heat of fusion of the ice which contains no salt. As a result, pure cold water and concentrated brine (6.9%) leaves the process as products. The term "brine" refers to water/salt solutions with salt concentrations above that of sea water.
a. Determine the flow rates of streams W and D when the feed is 1000 kg/h.
b. Determine the flow rates of streams C, B, and A as well.