Reference no: EM1341894
Question 1: The variation of EMF for the cell below with temperature is shown in the graph below.
Zn(s)| ZnCl2 (aqueous, conc. = 0.05 M) | AgCl(s) ,Ag(s)
(a) Write down the equation for the overall cell reaction
(b) For this cell reaction calculate the changes in free energy, entropy and enthalpy for this reaction at 298 K.
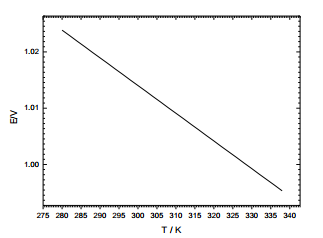
(a) Write down the equation for the overall cell reaction
(b) For this cell reaction calculate the changes in free energy, entropy and enthalpy for this reaction at 298 K.
Question 2: The W-cell consists of a cadmium amalgam anode and a mercury-mercurous sulfate cathode with a saturated cadmium sulfate solution as the electrolyte. This cell has been commonly used as a voltage standard in laboratories, replacing the previous standard C-cell. The voltage of this cell is 1.0183 V at 293 K and the temperature coefficient (dE/dT)p of the cell s –4.06x10-5 V K-1
a. Write down a cell diagram for the W-cell.
b. Write down the half cell and the full cell reactions.
c. Calculate ΔG at 20oC for the cell reaction.
d. Calculate ΔS at 20oC for the cell reaction.
e. The C-cell has an EMF defined by [1.434 - 0.0012 (T - 15)] Volts, where T is in oC. Calculate the temperature coefficient of the C-cell and suggest why the W-cell is better suited as a voltage standard than the C-cell.
f. Explain why when using standard cells no appreciable current should be drawn?
Question 3: (a) In a biotechnology laboratory using micro-scale techniques, it is necessary to transfer liquids by capillary by making use of capillary action. You have available clean glass capillaries of diameter 0.5 mm and 0.2 mm, and capillaries of diameter 0.5 mm made from a plastic which has a contact angle with water of 60o and from PTFE which has a contact angle with water of 100o
. Using appropriate calculation show which capillaries would be most useful for transferring the largest volume of water and why? Could surfactant addition to the water help in their transfer using capillaries?
(b) Two capillary tubes with inside diameters of 1.0 mm and 2.0 mm, respectively, are inserted into a liquid of density 0.95 g cm-3. Calculate the surface tension of the liquid if the difference between the capillary rises in the tubes is 6 mm. Take the contact angle to be zero.
Question 4: The following surface tensions were measured for an aqueous solution of a surfactant
CH3(CH2)9(OCH2CH2)5OH at 298 K:
The c.m.c. is at 5 x 10-4 mol dm-3.
(a) Rationalise the concentration dependence of the surface tension.
(b) Calculate the area occupied by each adsorbed surfactant molecule at the c.m.c..
(c) Describe another experimental method for determining the c.m.c..