Reference no: EM13982214
Solve Discussion Questions. I need prelab, write prelab in your words. make points or steps for what we would do in lab.
Group Discussion Questions
For the following spectrum, use the correlation chart provided earlier in this packet to assign all IR peaks to functional groups AND specific vibrating bonds, as appropriate
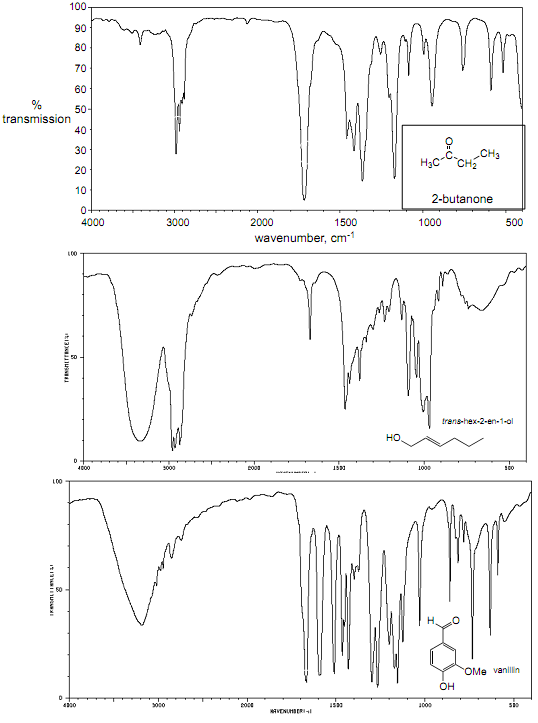
Infrared Lab: Pre-Lab
You MUST have the following Pre-lab completed in your notebook and checked by the TA at the beginning of lab! All parts of the Pre-lab must be handwritten IN INK. Include all of the following as part of your pre-lab assignment:
1) Title and date of the experiment
2) A completed version of the Table below. Look up the molecular structure, molecular weight, melting point and MSDS information.
| Name |
Molecular Structure |
Molecular Weight (g/mol) |
Melting Point (for solids Boiling Point (for liquids) (?C) with SOURCE |
MSDS (Hazards Identification) |
| Benzoic Acid |
|
|
|
|
| Biphenyl |
|
|
|
|
| 4-t-butylphenol |
|
|
|
|
| Hexane |
|
|
|
|
| 2-Propanol |
|
|
|
|
| Butylamine |
|
|
|
|
The information you will need to complete this table is available from several websites, a good one is the Sigma Aldrich website (www.sigmaaldrich.com). On the Sigma Aldrich site:
- Search for the compound
- The search will generate a list of different forms of the compound that the Sigma Aldrich sells.
- Choose the link for a pure form of the compound (not a solution, for example), and you should be able to find the compound's molecular structure, molecular weight, and melting point.
- Below the compound's name, there should be a button "MSDS". Click the button to get a pdf file of the Materials Safety Data Sheet. Scroll to Section 2.2 of the document ("Hazards Identification") and write down the relevant information under "Hazard Statement(s)".
3) The procedure of this lab packet, re-written and summarized briefly in your own words, IN INK (not pencil)
4) Complete the Table below using information in this packet
| Waste |
Where to Discard |
| Organic Chemicals |
1) Title and date of the experiment
2) Complete the Table
3) The procedure
4) complete the table of where to discard the waste.
?I want you solve only questions and and pre-lab
|
Pros and cons of hiring faculty from india
: 1. What are pros and cons of hiring faculty from India? 2. How many courses would you recommend that SUA should teach by offshoring faculty after five years?
|
|
When deciding whether to make or buy the component
: When deciding whether to make or buy the component, what cost of making the component should be compared to the price of buying the component?
|
|
Reneging on pension commitments
: Some years later, Nick sues Ralph for reneging on those pension commitments. What is the likely result of the lawsuit? Identify the relevant rule of law and explain, in detail, the arguments both parties will make, and the reason(s) for your concl..
|
|
What is steady state rate of heat flow through pane of glass
: What is the steady state rate of heat flow through a pane of glass that is 40.0 cm by 30.0 cm with a thickness of 4.00 mm when the outside temperature of the glass is -10.0oC and its inside temperature is 25.0oC? The thermal conductivity of glass i..
|
|
Assign all ir peaks to functional groups
: For the following spectrum, use the correlation chart provided earlier in this packet to assign all IR peaks to functional groups AND specific vibrating bonds, as appropriate
|
|
Purchase stock or bonds from a private company
: Assuming that everything else is equal, a U.S. government bond that matures 10 years from now most likely pays a interest rate than a U.S. government bond that matures 30 years from now.
|
|
Lp problem to minimize cost
: The cost for keeping these in inventory for 1 month is estimated to be $6 per tent for each tent left at the end of the month. Formulate this as an LP problem to minimize cost while meeting demand and not exceeding the monthly production capacity.
|
|
How much does the cable stretch under its own weight
: A steel cable with a cross-sectional area of 4.17 cm2 has a mass of 2.39 kg/m . If 356 m of the cable is hung over a vertical cliff, how much does the cable stretch under its own weight? The acceleration of gravity is 9.8 m/s 2 and Young's modulus..
|
|
How your opinion is different from that of the article
: Write one complete paragraph (must have a topic sentence, sentence transitions, and a point sentence) that sets up a quote from the article, frames it in context, and then identifies your position. You must distinguish how your opinion is differen..
|