Crude oil is separated into several fractions, each of these fractions are composed of many undefined hydrocarbon components. For simplicity, the crude oil is assumed to be a mixture of five real compounds and will be split into two streams only (top and bottom) using a distillation column. The components in the crude, amount of feed of each component and their normal boiling points are shown below.
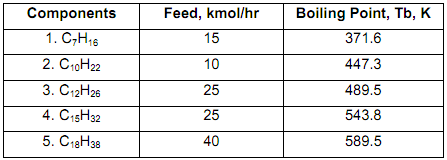
In the distillate, there can be a maximum of 8% of component 4 (C15H32) and in the bottom, there can be a maximum of 4% of component 3 (C12H26).
Question
Calculate:
(a) Minimum number of stages required for the separation
(b) Minimum reflux ratio for the separation
(c) Actual number of stages when the actual reflux ration is 1.4 times the minimum reflux ratio.
(d) Actual reflux ratio, when the actual number of stages is 17.
(e) If the actual reflux ratio is 50 times the minimum reflux ratio what happens to the actual number of stages. Comment on your results.
Pure component vapour vp P) pressure (bar) can be calculated using the following Antoine equation:
log10 Pvp = A - B / C + T - 273.15, where T is K.
The Antoine constants are shown in the following table for each component.
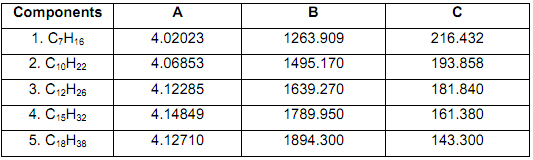
The Vapour Liquid Equilibrium (VLE) constants (ki) can be calculated by ki = Piv/P, where P is the total pressure (1 bar). The relative volatility (α, ir) of each component (i) with respect to a reference component (r) can be calculated by α i,r = ki/kr