Electron transfer is the easiest type of redox process, an example being
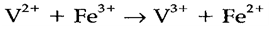
A majority of reactions of that type are very fast, but oxidation by some complexes (especially of CoIII) is much slower. In an inner sphere process, the coordination sphere of one complex is substituted by a ligand bound to the other complex, which then reacts as a bridge and can be transferred in the mean while the redox process. As isotopic labeling studies give that the oxidation of aqueous Cr2+ with [CoIII(NH3)5Cl]2+ proceeds via a bridged species Cr-Cl-Co, the
chlorine not exchanging with free labeled Cl- in solution but remaining collide to the kinetically inert CrIII product. An inner sphere mechanism needs one of the reactants to be substitutionally labile, and a ligand that can performs as a bridge. One test is to compare the rates of reaction with the ligands azide and (N bonded) thiocynanate NCS-; azide is usually better at bridging and so produces faster rates if the inner sphere route is operating.
The outer sphere mechanism involves no disruption of the coordination of either complex, and is always needs as a route to electron transfer unless the inner sphere rate is faster. The Marcus theory displays that the rate of outer sphere transfer depends on:
(i) the orbital communication between the two metal centers involved, a factor that decreases roughly exponentially with the distance between them;
(ii) the change in metal-ligand distances giving from electron transfer, the effect that gives most of the activation energy for the reaction;
(iii) an enhancement term, which effect on the difference of redox potentials of the two couples involved.