Ionization Isomerism
This is best displayed through an instance. 'CrCl3.6H2O' present in four solid forms, that dissolve in water to give dissimilar species:
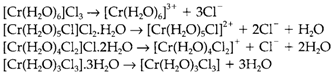
The dissimilar isomers all have an octahedral CrIII complex but the coordinated ligands are not similar; for instance, in the first case the three Cl- ions are exist in the crystal lattice of the solid compound but are directly not bound to the metal.