Geometrical isomerism
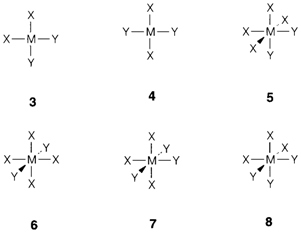
The truth that tetrahedrally coordinated compounds MX2Y2 comprise only one possible isomer was traditionally significant in establishing the structure of carbon compounds. While the coordination is square planar there are two probabilities, termed as the cis (3) and trans (4) forms. Geometrical isomers take place also in octahedral complexes: with MX2Y4 the two isomers are also called cis (5) and trans (6), and for MX3Y3 the words mer (7 from 'meridional') and fac (8 from 'facial') are employed.
Geometrical isomerism can also consider to the possibility of dissimilar coordination geometries, even though these are rather uncommon. Square-planar or tetrahedral coordination is, technically, possible with CN=4, and an instance with CN=5 happens with [Ni(CN)5]3-, that can accept shapes approximating either to a trigonal biyramid (the generally supposed shape;) or a square pyramid (9).
