Q. Show the properties of Lithium Aluminium Hydride?
Lithium aluminium hydride is much more useful than aluminium hydride. It is prepared in the laboratory by the action of LiH on AlCl3 in diethyl ether:
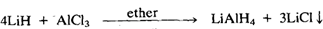
Lithium aluminium hydride is a greyish-white solid which decomposes into its elements above 400 K. It' is stable in dry air but reacts violently with water:
LiAlH4, + 4H20--------------------------> LiOH + AI(OH)3, + 4H2
LiAlH4 is readily soluble in ethers. It is a very important reducing agent for both inorganic and organic compounds. It reduces inorganic halides to hydrides, e.g.:
4BCl3 + 3LiAlH4---------------> 2B2H6, + 3LiCI + 3AlC13
MCI4, + LiAlH4, -- ----------------->-+ MH4, + LiCl + AICI3,
(M Si, Ge, Sn)
LiAlH4, is one of the most important reducing agents in organic chemistry because it reduces as many as 60 functional groups including ethylenic >C=C< double bonds. But, it is less selective than sodium borohydride. Before we discuss the halides of boron and aluminium, you may like to attempt the following SAQ based on the hydrides of boron and aluminium.