Q. Show the d-orbital energy level splitting?
Let us consider one of the consequences of the d-orbital energy level splitting. [Ti (H2o6)3+ is a d1 system meaning that the central metal ion, Ti3+ has one electron in d-orbital. In a free ion this one electron could be found in any one of the d-orbitals since all the d-orbitals are equal in energy. However, in the complex [Ti (H2o6)3+ the electron would prefer to go into any one of the t2g set of orbitals since they are lower in energy. By doing so it will lower the energy of the system by 2/5 D0 as compared to the energy of the Ti3+ ion in a uniform field. This difference in energy between the ion in a uniform field and the complex is called Crystal Field Stabilisation Energy (CFSE). If there are two electrons present in t2g set of orbitals, the CFSE would be equal to 2 x (-2/5D0) or -4/5D0. Hence, we can calculate the CFSE for any complex provided we know the electronic distribution.
Consider a complex, Fe (III)L6, which belongs to the d5 system. In a fe3+ ion in a spherically uniform field the five d-electrons will go into five different orbitals with parallel spins according to Hund 's rules. as shown in Fig. (a). However, when it is surrounded by six, the five d-electrons will distribute themselves in either of the following two ways,
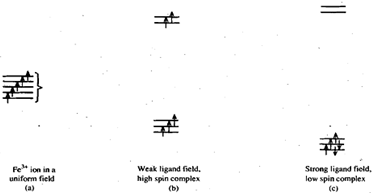
Fig represents the complex in high spin state with five unpaired electrons while Fig. (c) shows the low spin configuration. These conclusions are the same as obtained by valence bond theory with a slight difference. The Scent theory gives the reason for the existence of the two types of complexes and can also predict which of the two configurations will be more stable in a particular case provided certain parameters are known. These parameters are A crystal field splitting energy and P, the pairing energy. Whenever we put an electron in eg set of orbitals, the system gets destabilised by energy equal to 3/5D0. On the other hand, by putting two electrons in the same orbital, the system again gets destabilised by an amount equal to pairing energy P. The decision as to which of the two configurations, i.e., high spin or low spin is more stable is rather easy to make. If the pairing energy is greater than then the two electrons will prefer to go in different orbitals with parallel spins giving a high spin complex. The reverse kill be true for a low spin complexes. Thus, we can write
if P > d, ------ high spin complex
P c A,, ------ low spin complex
p = g ------ the two states are in equilibribm.