Define the Cell potential of a galvanic cell?
The cell potential of a galvanic cell is the electric potential difference between terminals of the same metal, and is defined by
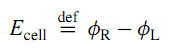
The subscripts R and L refer to the right and left terminals. The equilibrium cell potential, Ecell, eq, is the cell potential measured under conditions of zero current when the cell is assumed to be in an equilibrium state.
Over a relatively long period of time, the state of an isolated galvanic cell is found to change. Nevertheless, the assumption of an equilibrium state is valid if the changes are very slow compared to the period during which we measure Ecell.
The long-term changes can be of two types. If there is a liquid junction between electrolyte solutions of different composition, slow diffusion of ions through the junction is inevitable. In a cell without a liquid junction, the reactants of the cell reaction can react directly without the passage of an electric current. For instance, in the cell of Figure the electrolyte solution is saturated with respect to gaseous H2 and solid AgCl, and therefore contains very small concentrations of dissolved H2 molecules and AgC ions. The direct reaction H2+2Ag+ → 2H+ + 2Ag occurs irreversibly and continuously in the solution, but is slow on account of the low concentrations. It is entirely arbitrary whether we show a particular electrode at the left or the right of the cell diagram, although often there is a preference to place the electrode attached to the positive terminal at the right. If we exchange the positions of the two electrodes in the diagram, then we must reverse the reaction equations for the electrode reactions and the cell reaction.
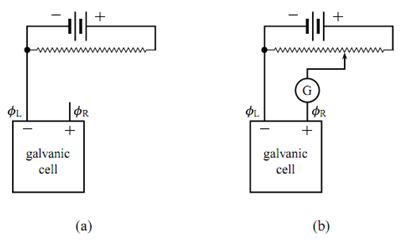
Figure: Potentiometer to measure the zero-current cell potential of a galvanic cell. (a) Galvanic cell with zero current. (b) Galvanic cell included in potentiometer circuit; G is a galvanometer.
reaction should show reduction at the right electrode and oxidation at the left, we must now write it as
Cu + Zn2+(aq) → Cu2C(aq) + Zn
even though the arrow is not in the direction of the reaction that actually occurs spontaneously. In other words, the cell reaction is written according to the cell diagram, not according to the direction of the spontaneous change.