Reference no: EM131248726
Question 1:
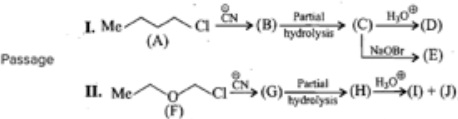
Compound (A) an alkaline with molecular formula (C5H10), exists. in vallous structures and stereoisomers. On monochiorination an ddichlorination. It again shows various structure and stereisomers.
Another isomer(II) on monochlorination gives two products. Th esiomer(II) is:
Doubt: Why not option B is the answer? Your response-
Question 2:
A hydrated sulphate of the metal contained 8.1% metal and 43.2% of SO42- ion by weight. The specific heat of the metal 0.24 cal g-1.
Number of moles of SO42- ion in one mole of the salt is
A 2
B 4
C 3
D 1
Doubt: Please explain with a detailed solution. Your response-
Question 3:
For the reaction system 2NO(g) + (O2) → 2NO2(g): Volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO, the rate of reaction will -
A. Diminish to one fourth of its initial value.
B. Diminish to one eight of its initial value.
C. Increase to eight times of its initial value.
D. Increase to four times of its initial value.
Doubt: How concentration become 8 times if volume is half and concentration is doubled? Your response-
Question 4:
Equivalent weight of postassium permanganate in alkaline medium is
molecular weight/1
molecular weight/3
molecular weight/5
molecular weight/6
Doubt: Please give solution if same is in acidic medium. Your response-
Question 5:
Which of the following has been arranged in circler of increasing covalent character?
KCI < CaCl2 < AlCl3 < SnCl4
SnCl4 < AICl3 < CaCl2 <KCI
AICl3< CaCl3<KCL<SnCl4
CaCl2 < SnCl4 < KCl < AlCl3
Doubt: Why is SnCl4 more covalent than all others?
Your response-
Question 6:
Each unit cell of NaCl consists of 14 chloride ions and ____.
A 13Na+ ions
B 14Na+ ions
C 6Na+ ions.
D 8 Na+ ions
Doubt: Electrical neutrality should be there, so answer should be 14. Please explain me how the answer is 13? Your response-
Question 7:
In which of the following pairs. The minerals are converted in to metals by self-reduction process?
Cu2S, PbS
PbS, HgS
PbS, ZnS
Ag2S, Cu2S
Doubt: Why not the answer is ZnS and Ag2S?
Your response-
Question 8:
Which statement is wrong about the hydrolysis of R - N⊕ ≡ C??
A Isocyaniders are hydrolysed with aqueous dilute acids but bot by alkali
B. U=ln th ehydrolysis of (R - N⊕ ≡ C?), first electrophile(H⊕) adds on the C atom and then nucleophile (OH⊕ adds to the same C atom.
C On partial hydrolysis (RNC. Gives N-substituted methanamide or formamide)
D On complete hydrolysis (RNC) gives 1o amine and RCOOH.
Doubt: How option B is correct? Please give proper explanation. Your response-
Question 9:
Li has following abnormal behavior in its group.
A:1 Lithium Carbonate decomposes into its Oxide on heating unlike other elements.
B: LiCl is covalent in nature.
C Li3N is a stable compound.
D. LiCl is a poor conductor of electricity in the molten state.
Doubt: Option d is also right as LiCl is covalent in nature, hence it should be a poor conductor of electricity in molten state.
Question 10:
In the long from of the periodic table, the valance shell electronic configuration of 5s25p4 corresponds to the element present in:
A Group 16 and period 6
B Group 17 and period 6
C Group 16 and period 5
D Group 17 and period 5
Doubt: The JEE Main have officially claimed that the answer for this question is option "B", i.e., Group 17 and period 6.
Can you please re-confirm, as I need to challenge the same since I had also opted for the same answer as mention by you.
|
Draw a project network diagram
: Assuming that one worker is required for each activity, prepare a resource-leveled schedule. What is the maximum number of workers required to finish the project on time?
|
|
Explain possible risks and barriers that pose a threat
: Explain possible risks and barriers that pose a threat to the primary solution and explain how to minimize the threats. As a contingency plan, propose 1 alternative solution to the business dilemma for each course outcome based on the findings in t..
|
|
Fully organic energy drink
: Assume you are a recent start-up company that manufactures and markets a new, fully organic energy drink. Draw up a simple plan for the selection / assessment of foreign target markets by outlining a set of criteria you intend to apply.
|
|
Plant research project - thyme herbal plant
: Plant Research Term Project Part: Scientific and Medical Evidence. MY PLANT: THYME HERBAL PLANT. Herbs and plants contain chemical compounds; some of them are effective at curing conditions, some are dangerous and others are a waste of effort and m..
|
|
Which statement is wrong about the hydrolysis
: How concentration become 8 times if volume is half and concentration is doubled - In the long from of the periodic table, the valance shell electronic configuration of 5s25p4 corresponds to the element present
|
|
Select a business of interest
: Write a report addressing a quantitative analysis (QA) project. Here, you are asked to select a business of interest and develop QA best practices that can be developed and implemented to increase revenues and/or to decrease costs. Please provide ..
|
|
Find the fourier series of the square wave
: Find the Fourier series of the square wave with period T defined on 0 ≤ t ≤ T by and extended periodically for all
|
|
List the critical path activities and project duration
: Assuming that one worker is required for each activity, prepare a resource-leveled schedule. What is the maximum number of workers required to finish the project on time?
|
|
Marketing is the social and managerial process
: What is the difference between want and need? Marketing is the social and managerial process?
|