Reference no: EM13840220
1. Which of the following is trigonal planar?
A. boron trifluoride, BF3
B. methyl anion, CH3
C. methane, CH4
D. ammonia, NH3
2. Which atomic orbitals overlap to form the C=O bond of 2-propanone?
A. C sp3 + O sp2
B. C sp2 + O p
C. C sp2 + O sp2
D. C sp3 + O sp
Draw these orbitals here:
3. Which of the following resonance structures makes the largest contribution to the structure of [H2CCHO]-? Explain.
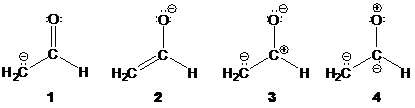
A. 1
B. 2
C. 3
D. 4
Explain here:
4. Identify the condensed formula of the following structure:
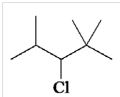
A. (CH3)2CHCHClCH(CH3)2
B. CH3CH(CH3)CHClCH(CH3)2
C. (CH3)2CHCHClC(CH3)3
D. (CH3)3CCHClCH(CH3)3
5. The most stable resonance contributor of this would be:
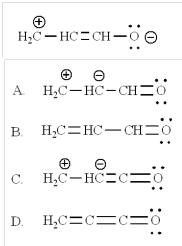
A. A
B. B
C. C
D. D
Show all resonance structures and provide explanation for the major contributor:
6. Does the equilibrium of this reaction lie to the left or right? Explain.
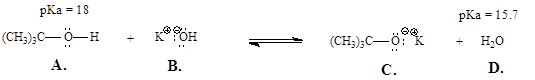
A) Left
B) Right
C) It cannot be determined.
D) The forward and reverse reactions are equally favored.
Provide explanations here:
7. What should go in the box on the right side of the equation? Show work indicating the direction of equilibrium of the reaction.
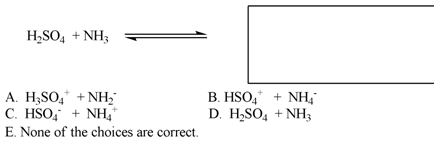
A) A
B) B
C) C
D) D
E) E
Show work here:
8. Rank the following from the weakest to the strongest base.
A) OH- < Cl- < NH2-
B) Cl- < NH2- < OH-
C) NH2- < OH- < Cl-
D) Cl- < OH- < NH2-
E) NH2- < Cl- < OH-
Provide explanation here: