Reference no: EM131102433
1. Which is the most acceptable electron dot structure for N2H2?
2. How many lone pairs of electrons are on the Xe atom in XeF6?
a. 1
b. 2
c. 0
d. 3
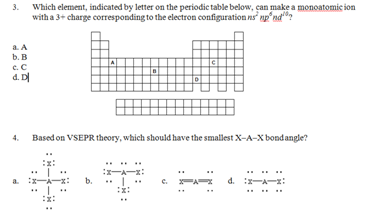
3. Which element, indicated by letter on the periodic table below, can make a monoatomic ion with a 3+ charge corresponding to the electron configuration ns2 np6 nd10?
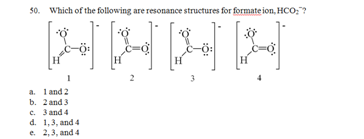
7. If two or more species have the same number of electrons, resulting in similar Lewis structures, they are said to be .
a. isoelectronic
b. resonant structures
c. ionic
d. neutral
e. covalent
8. What is the formal charge on each atom in chloroform, CHCl3?
a. C atom = 0, H atom = 0, and each Cl atom = 0
b. C atom = 0, H atom = +1, one Cl atom = -1, and two Cl atoms = 0
c. C atom = +4, H = -1, and each Cl atom = -1
d. C atom = +2, H = +1, and each Cl atom = -1
e. C atom = -4, H = +1, and each Cl atom = +1
9. How many structures (i.e., unique isomers) exist for dichlorobenzene?
a. 2
b. 3
c. 4
d. 5
e. 6
10. If 2.300 g of a polymer sample is burned in excess oxygen, it produces 2.955 g H2O and 7.217 g CO2. What is the empirical formula of this polymer? This polymer consists only of carbon and hydrogen.
a. CH
b. CH2
c. C2H3
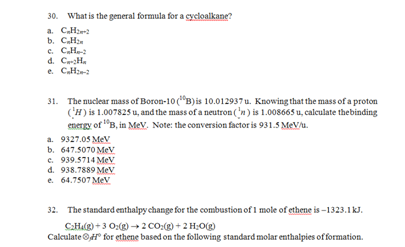
d. C5H8
e. C7H811. Which of the following elements is able to form a molecular structure that exceeds the octet rule?
a. Ne
b. B
c. O
d. F
e. I
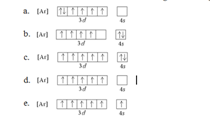
12. What is the correct orbital box diagram for the ground state electron configuration of Cr?
13. Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of sulfur dioxide, SO2.
a. The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
b. The electron-pair geometry is trigonal-planar, the molecular geometry is bent.
c. The electron-pair geometry is tetrahedral, the molecular geometry is bent.
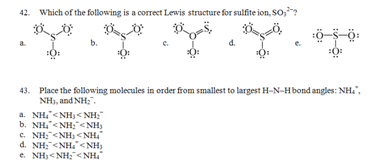
d. The electron-pair geometry is tetrahedral, the molecular geometry is linear.
e. The electron-pair geometry is trigonal-bipyramidal, the molecular geometry is linear.14. Which one of the following molecules is polar?
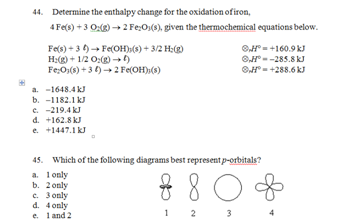
a. CO2
b. SF4
c. XeF2
d. XeF4
e. SO3
15. Which of the following statements is/are CORRECT?
1. The overlap between an s orbital and a p orbital is called a pi-bond.
2. The overlap of two s orbitals in H2 is called a sigma bond.
3. HF is formed from the overlap of a hydrogen 1s orbital with a fluorine 2s orbital.
a. 1 only
b. 2 only
c. 3 only
d. 2 and 3
e. 1, 2, and 3
16. What is the molecular geometry around a central atom that is sp3 hybridized and has two lone pairs of electrons?
a. Bent
b. Linear
c. trigonal-planar
d. trigonal-pyramidal
e. trigonal-bipyramidal

What mass of SO2 is produced from the combustion of 0.331 g P4S3?
a. 0.00150 g
b. 0.00451 g
c. 0.0321g
d. 0.0964 g
e. 0.289 g
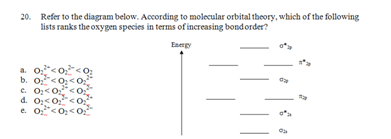
21. If a nucleus undergoes a negatron emission?2s
a. its atomic number decreases by one and its mass number is unchanged.
b. its atomic number is unchanged and its mass number increases by one.
c. its atomic number increases by one and its mass number is unchanged.
d. both its atomic number and its mass number are unchanged.
e. its atomic number is unchanged and its mass number decreases by one.
22. Structural isomers are compounds that have
a. the same molar masses, but different elemental composition.
b. have identical structures, but contain different isotopes of the same elements.
c. two or more resonance structures.
d. the same elemental composition, but the atoms are linked in different ways.
e. the same physical properties, but different chemical properties.
23. Use Lewis structures to predict the bond order for a sulfur-oxygen bond in sulfur dioxide.
a. 1/2
b. 1
c. 4/3
d. 3/2
e. 2
24. Which of the following alcohols is likely to be least soluble in water?
a. 3-pentanol
b. 2-butanol
c. 1,2-ethanediol
d. 1,2,3-propanetriol
e. methanol
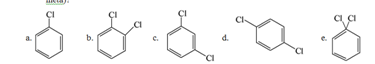
25. Which of the structures below has the common name m-dichlorobenzene (where m- is meta)?
26. What class of compounds is responsible for many of the distinctive odors of artificial flavors and perfumes?
a. ethers
b. aldehydes
c. Esters
d. amides
e. alkenes
27. Complete the following nuclear reaction.
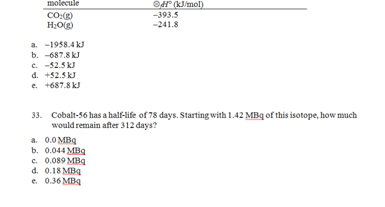
34. Enriched uranium is uranium that has a greater proportion of .
a. cadmium-112
b. uranium-235
c. uranium-238
d. radium-226
e. plutonium-248
35. The Becquerel (Bq) is an SI unit for the measurement of radiation. One Bq represents:
a. 1 J energy absorbed per kg of tissue.
b. 3.7 ? 1010 disintegrations per second.
c. 1 disintegration per second.
d. 1 J energy absorbed per kg of matter.
e. 1 calorie energy absorbed per kg of tissue.
36.Which of the following atoms contains the largest number of neutrons

37. Which of the following statements concerning ionic compounds is/are correct?
1. As ion charges increase, the attraction between oppositely charged ions increases.
2. Although not electrically conductive like metals, ionic compounds are malleable.
3. Positive and negative ions are attracted to each other by electrostatic forces.
a. 1 only
b. 2 only
c. 3 only
d. 1 and 3
e. 1, 2, and 3
38. A precipitate will form when aqueous Pb(NO3)2 is added to an aqueous solution of .
a. Cu(NO3)2
b. CaBr2
c. NaCH3CO2
d. Ca(ClO4)2
e. NaNO3
39. Which molecule in the reaction below is the reducing agent? 2 C2H6(g) + 7 O2(g) ? 4 CO2(g) + 6 H2O(g)
a. C2H6
b. O2
c. H2O
d. CO2
e. None
40. How much energy (in kJ) is required to change the temperature of 1.00 kg Fe from 25.0 oC to 1515 oC? The specific heat capacity of iron is 0.449 J/g•K. Note: q = Cp m ΔT.
a. 1.49 kJ
b. 3.32 kJ
c. 66.9 kJ
d. 669 kJ
e. 3.32 ? 106 kJ
41. All of the following are oxidation-reduction reactions EXCEPT
a. CaCO3(s) ? CaO(s) + CO2(g)
b. 2 Na(s) + Br2(g) ? 2 NaBr(g)
c. Fe(s) + 2 HCl(aq) ? FeCl2(aq) + H2(g)
d. C(s) + O2(g) ? 2 CO(g)
e. 2 ) ? 2 H2(g) + O2(g)
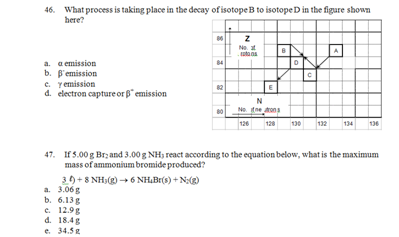
48. All of the following statements concerning nuclei are true EXCEPT
a. only hydrogen-1 and helium-3 have more protons than neutrons.
b. from He to Ca, stable nuclei have roughly equal numbers of protons and neutrons.
c. isotopes with a low neutron to proton ratio always decay by alpha particle emission.
d. the neutron to proton ratio in stable nuclei increases as mass increases.
e. beyond calcium, the neutron to proton ratio is always greater than one for stable nuclei.
49. If 50.0 g of ethanol, CH3CH2OH, at 20.0 °C absorbs 1.45 kJ of heat, what is the final temperature of the ethanol? The specific heat capacity of ethanol is 2.44 J/g?K.
a. 8.1 ?C
b. 21.2 ?C
c. 31.9 ?C
d. 47.7 ?C
e. 90.8 ?C