Reference no: EM13873915
Need solutions for the three questions below. I am in a second year biochemistry class.
1). Consider the following two reactions:
ADP + Pi -> ATP ΔGo' = 30.5 kJ/mole
Creatine + Pi -> Creatine phosphate ΔGo' = 43.1 kJ/mole
The enzyme creatine kinase can transfer the phosphate from ATP to creatine, thus generating creatine phosphate. Creatine phosphate can be used as a source of phosphate in muscle cells.
Write the equation for the reaction in which creatine phosphate is synthesized (as described above). What is the ΔGo' for this reaction? Would you consider this reaction to spontaneous or non-spontaneous at standard state?
In a recovering muscle cell, the following concentrations are observed:
|
Metabolite
|
Concentration (mM)
|
|
ATP
|
8.0
|
|
ADP
|
0.02
|
|
Pi
|
3.0
|
|
Creatine
|
9.0
|
|
Creatine phosphate
|
16.0
|
Calculate the ΔG' for creatine phosphate formation in these cells at 37oC. Is the reaction spontaneous in recovering muscle cells?
Is the ΔG' calculated in b) different from the ΔGo' that you calculated in a)? Why or why not?
During the first few seconds of heavy exercise in muscle cells, the ATP levels can drop to 0.8 mM and the ADP levels can increase to 0.2mM. What effect would this have on the spontaneity of the reaction in b)? What would you predict to happen to the reaction in a)? What is the significance of this?
2) . You're a biochemist hired by NASA to study the results of a probe sent to Rhea, an earth sized planet several light years away. There is a large amount of excitement over Rhea as it contains several primitive life forms resembling plants that contain carbohydrates and proteins! It is currently hypothesized that these "plants" could provide a source of nutrients for any future manned missions to Rhea. The most recent "plant" data from the probe is listed below:
Carbohydrates:
Two disaccharides detected:
α-L-glucopyranose linked to β-L-fructofuranose in an α1-β2 arrangement.
β-L-galactopyranose linked to α-L-glucopyranose in an α1-4 arrangement.
Protein amino acid composition:
D-glycine 7.5%
D-alanine 7.2%
D-serine 5.7%
... More data in next transmission.
Armed with this, you dash into your supervisors' office!!!
What have you learned from this latest dataset from the probe? Why is it important?
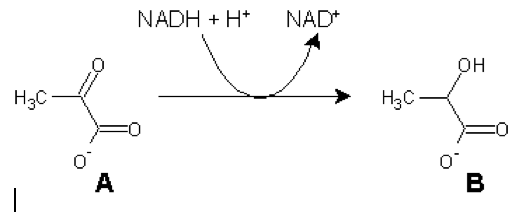
3). Given the following reversible reaction:
<see attachment>
Identify A and B, as well as the enzyme that catalyzes this reaction (no peeking now... you can't peek during the exam).
What is the significance of this reaction? When would it occur (give an example)?
Suppose the ΔGo' of the above reaction is -25 kJ/mole. What is the highest ratio of products to reactants that would allow this reaction to go forward in the cell?
There are some very rare cases where the enzyme catalyzing the above reaction is mutated and inactive. These patients suffer from exertional myoglobinuria (the presence of myoglobin in the urine during exercise) and fatigue upon heavy exercise. Explain these symptoms (hint: Where is myoglobin normally found?).
|
Transport across biological membranes
: Which of the following is NOT relevant to facilitated transport across biological membranes? Which of the following is NOT relevant to facilitated transport across biological membranes?
|
|
What is the expected return on this stock
: Sugar and Spice stock is expected to produce the following returns given the various states of the economy. What is the expected return on this stock?
|
|
Environmental effects be dealt with when evaluating project
: An electric utility is considering a new power plant in northern Arizona. Power from the plant would be sold in the Phoenix area, where it is badly needed. Because the firm has received a permit, the plant would be legal; but it would cause some air ..
|
|
Examine the future of health care in the united states
: Examine the future of health care in the United States. Look back to your own definition of the healthcare system. How would you define it
|
|
Which creatine phosphate is synthesized
: Write the equation for the reaction in which creatine phosphate is synthesized (as described above). What is the ΔGo' for this reaction? Would you consider this reaction to spontaneous or non-spontaneous at standard state?
|
|
Following is a trial balance for the jayce county general
: Following is a trial balance for the Jayce County General Hospital, a governmental hospital. The hospital does not use fund accounting.
|
|
Differences between variable and absorption costing
: What are the differences between variable and absorption costing? Why is variable costing not allowed for GAAP reporting? Which method is more useful for internal decision making? Why? As a manager, which would you prefer? Why?
|
|
Evaluate the results of theseratios
: Comparative financial statements of the Boeckman Company for 2009 and 2010 are as follows:
|
|
What is the fraction of protein with ligand
: 8. While working at a biotech startup, you isolate an inhibitor against an important drug target, the dimeric protein known as Enzyme X. Answer the following: a. The binding of your inhibitor to Enzyme X appears to involve an allosteric mechanism and..
|