Reference no: EM13857243
1. True or False: The more shells in an atom, the lower its electronegativity. Explain.
A. Always true; the greater the number of shells in an atom, the lower the electronegativity.
B. Always false; the greater the number of shells in an atom, the higher the electronegativity.
C. Sometimes true and sometimes false; This holds true only within any one atomic group (vertical column).
D. Sometimes true and sometimes false; This holds true only across any given period (row) of the periodic table.
2. Which of the following molecules would contain a dipole?
A. H-F
B. Cl-Cl
C. H-H
D. F-F
E. All of these
3. Which of the following molecules has the highest boiling point?
Question 3 options:
A. BH3
B. NH3
C. CH4
D. SH2
E. All of these have the same boiling point.
4. If you need 3.01 × 1023 molecules of sucrose, how many liters of a 4.00 molar solution would you need?
A. 0.125 L
B. 0.250 L
C. 4.00 L
D. L
E. None of these
5. A sealed plastic bottle is filled with enough sand so that the bottle floats just beneath the surface in ocean water. Some sand is then removed and the bottle is then placed in some fresh water where it floats just beneath the surface. What is true about the amount of sand that was removed from the bottle?
Question 12 options:
A. The mass of sand removed divided by the volume of the original plastic bottle equals the density of the ocean water.
B. The mass of the sand removed divided by the mass of the sand remaining in the plastic bottle is the same ratio as the density of fresh water to the density of ocean water.
C. The amount of sand removed from the plastic bottle equals the amount of salt dissolved in the ocean water.
D. The mass of the sand removed multiplied by the density of ocean water equals the volume of the plastic bottle.
6. Which of the following best describes a two-molar sucrose solution?
A. one liter of solution that contains 2 moles of sucrose
B. one liter of solution that contains 2 moles of water
C. one liter of solution that contains 6.02 × 1023 molecules of sucrose
D. two liters of solution that contains 1 mole of sucrose
E. one mole of sucrose dissolved in 2 liters of solution
7. If you were somehow able to sit at the surface of an ice cube and were able to slow the movement of water molecules from the solid to the liquid phase, what would eventually happen to the ice cube?
A. It would increase in size.
B. It would melt because you are sitting on it and you give off heat.
C. It would melt because you have slowed the rate of water uptake.
D. It would melt because you have increased the rate of water uptake.
E. None of these
8. Which of the following bonds would be the most polar?
A. C-F
B. C-Cl
C. C-Br
D. C-I
E. All are equally polar.
9. What is the difference between a dipole-dipole interaction and an ion-dipole interaction?
A. one involves dipole attraction between neutral molecules while the other involves dipole interactions with ions
B. one involves hydrogen bonding while the other does not
C. one involves salts and water while the other doesn't involve water
D. one involves ionic molecules interacting with other ionic molecules while the other deals with polar molecules
E. None of these
10. Which of the above liquids has the strongest adhesive forces?
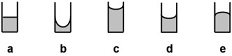
11. Which of the following is a correctly balanced equation?
A. P4 + 6 H2 4 PH3
B. 1 P4 + 6 H2 4 PH3
C. 0 P4 + 6 H2 4 PH3
D. 2 P4 + 12 H2 8 PH3
E. P4 + 3 H2 PH3