Reference no: EM13857359
1. Consider the combustion of acetic acid CH3COOH(g) + 2O2(g) → 2H2O(g) + 2CO2(g). The following data are for T = 298.15 K and standard state:
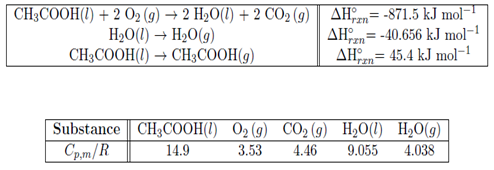
(a) Calculate ΔHorxn (T = 391.4 K) for the combustion of glucose. Assume the heat capacities above are constant over the necessary temperature range.
(b) Calculate/estimate ΔUorxn at T = 391.4 K.
2. A mixture of ethanol and water is often used in a sponge bath to cool individuals with high temperatures. Ethanol is added to aid in the evaporation process which is primarily responsible in the cooling. What volume of ethanol (measured and applied at 25°C) is required to remove 450. kJ from a person with a temperature of 104°F?
3. A piece of Zn metal of mass 65.38 g at a temperature 25°C and an ambient pressure of 1 bar is dropped into a water bath maintained at 98°C. The Zn undergoes a volume change from 9.16 mL to 9.22 mL as it equilibrates. What is ΔH - ΔU for this process?
4. Adiabatic process is quite important in atmospheric chemistry and climate. Large air masses called parcels have large volume to surface area ratios which make heat transfer between the such parcels and the surrounding atmosphere inefficient. Adiabatic processes are thought to play a critical role in several weather patterns including anomalous winds and hurricanes.
A "chinook" is a weather event observed where in continental North America where the Rocky Mountains meet the Great Plains. Chinooks are warming winds, see the figure resulting from a moist air mass forced to rise over the windward side of the mountains. As moist air is forced to higher, colder altitudes, the water in the air condenses creating precipitation on the windward side of the mountain. The leftover dry air continues over to the leeward side of the mountain and falls down in altitude into the plain. As the dry air falls, it undergoes an adiabatic compression which gives rise to the chinook. A chinook descends from the height of the 14000' where the atmospheric pressure is 420 Ton and ambient temperature is -15°C. The chinook descends to an altitude of 3000' where the atmospheric pressure is 690 Torr. Assume the chinook is composed of ideal air with cvm = 0.72 kJ kg-1 K-1 or 20.7643 kJ mol-1 K-1, and that it descends slow enough down the mountain so that the process is approximately reversible.
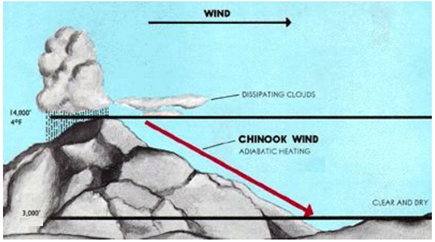
(a) (Hard, see Atkins if you have trouble) What is the temperature of the Chinook wind at 3000'?
(b) Compute the following quantities for the Chinook wind (in kJ kg-1):
- Q, heat absorbed by chinook over the process.
- ΔU.
- Wby, work done by the Chinook.
(c) Explain, in terms of the 1st Law of Thermodynamics, why the temperature of the Chinook rose during the descent.
|
Product-price-promotion and place
: When it pertains to service organizations, how does technological advancement effect the four P's? (Product, Price, Promotion, Place). Even a small summary of each will help get me started on understanding
|
|
How loyalty may positively impact teams performance-hence
: Discuss at least three attributes of a team leader that may lead to team's loyalty to him and the organization. Also discuss how loyalty may positively impact team's performance and hence, organizational success.
|
|
What are likely to be the best measures of actual
: What are likely to be the best measures of actual and potential value for EACH of the listed customer bases?
|
|
Inspected the number of defective plastic models
: A production manager at a Contour Manufacturing plant has inspected the number of defective plastic models in 5 random samples of 20 observations each.
|
|
What volume of ethanol is required
: A mixture of ethanol and water is often used in a sponge bath to cool individuals with high temperatures. Ethanol is added to aid in the evaporation process which is primarily responsible in the cooling. What volume of ethanol (measured and applie..
|
|
Customer relationship management systems
: Evaluate how supply chain management systems, enterprise resource planning systems, and customer relationship management systems are currently used, or could be used, within the organization. Organization is Tesla.
|
|
Prepare a list of internal and external stakeholders
: Prepare a list of internal and external stakeholders for a health organization in preparation of strategic planning; categorize each group.
|
|
Dichotomous outcome has only two possibilities
: A dichotomous outcome has only two possibilities. The probability of two independent events occurring together equals the product of each of the individual event probabilities. A probability is found by dividing the number of possible outcomes (o) b..
|
|
Part of the contemporary business and government community
: 1. Why has connectivity become such an important part of the contemporary business and government community? 2. What should be the strategic thrust of RIM with the Blackberry over the next five years?
|