Reference no: EM13493535
1.IDX Technologies is a privately held developer of advanced security systems based in Chicago. As part of your business development strategy, in late 2008 you initiate discussions with IDX’s founder about the possibility of acquiring the business at the end of 2008. Estimate the value of IDX per share using a discounted FCF approach and the following data:
¦ Debt: $30 million
¦ Excess cash: $110 million
¦ Shares outstanding: 50 million
¦ Expected FCF in 2009: $45 million
¦ Expected FCF in 2010: $50 million
¦ Future FCF growth rate beyond 2010: 5%
¦ Weighted-average cost of capital: 9.4%
2.Sora Industries has 60 million outstanding shares, $120 million in debt, $40 million in cash, and the following projected free cash flow for the next four years:
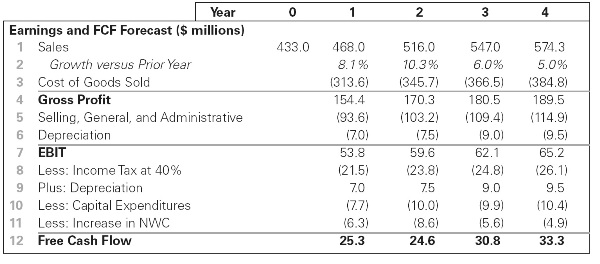
a. Suppose Sora’s revenue and free cash flow are expected to grow at a 5% rate beyond year 4. If Sora’s weighted average cost of capital is 10%, what is the value of Sora’s stock based on this information?
b. Sora’s cost of goods sold was assumed to be 67% of sales. If its cost of goods sold is actually 70% of sales, how would the estimate of the stock’s value change?
c. Let’s return to the assumptions of part (a) and suppose Sora can maintain its cost of goods sold at 67% of sales. However, now suppose Sora reduces its selling, general, and administrative expenses from 20% of sales to 16% of sales. What stock price would you estimate now? (Assume no other expenses, except taxes, are affected.)
*d. Sora’s net working capital needs were estimated to be 18% of sales (which is their current level in year 0). If Sora can reduce this requirement to 12% of sales starting in year 1, but all other assumptions remain as in part (a), what stock price do you estimate for Sora? (Hint: This change will have the largest impact on Sora’s free cash flow in year 1.)
3.Consider the valuation of Kenneth Cole Productions in Example 9.7.
a. Suppose you believe KCP’s initial revenue growth rate will be between 4% and 11% (with growth slowing in equal steps to 4% by year 2011). What range of share prices for KCP is consistent with these forecasts?
b. Suppose you believe KCP’s EBIT margin will be between 7% and 10% of sales. What range of share prices for KCP is consistent with these forecasts (keeping KCP’s initial revenue growth at 9%)?
c. Suppose you believe KCP’s weighted average cost of capital is between 10% and 12%. What range of share prices for KCP is consistent with these forecasts (keeping KCP’s initial revenue growth and EBIT margin at 9%)?
d. What range of share prices is consistent if you vary the estimates as in parts (a), (b), and (c) simultaneously?
4.You notice that PepsiCo has a stock price of $52.66 and EPS of $3.20. Its competitor, the Coca-Cola Company, has EPS of $2.49. Estimate the value of a share of Coca-Cola stock using only this data.
5.Suppose that in January 2006, Kenneth Cole Productions had EPS of $1.65 and a book value of equity of $12.05 per share.
a. Using the average P/E multiple in Table 9.1, estimate KCP’s share price.
b. What range of share prices do you estimate based on the highest and lowest P/E multiples in Table 9.1?
c. Using the average price to book value multiple in Table 9.1, estimate KCP’s share price.
d. What range of share prices do you estimate based on the highest and lowest price to book value multiples in Table 9.1?