Reference no: EM131072851
1. A 5.00 g sample of a brownie with nuts is burned in a bomb calorimeter containing 2025 g of water. The temperature of the water increases from 23.50°C to 33.47°C. How much heat, in joules, did the brownie release when it burned? (Cwater = 4.184 J/g°C)
A) 1.99 x 105 J D) 8.45 x 104 J
B) 2.80 x 105 J E) 7.00 x 102 J
C) 4.92 x 102 J
2. Which of the following compounds is a ketone?
A) CH3CH2OH D) CH3CH2COCH3
B) CH3CH2CO2H E) CH3CHO
C) CH3OCH3
3. Iron metal reacts with hydrochloric acid as follows:
2Fe(s) + 6HCl(aq) → 2FeCl3(aq) + 3H2(g)
If 22.4 g of iron react with excess HCl, and 59.4 g of FeCl3 are collected, what is the percent yield of the reaction?
A) 65.0% B) 109% C) 91.4% D) 73.0% E) not enough information given
4. In the process of obtaining lead from PbS, or galena, the galena is "roasted" (heated in the presence of oxygen), so that the following reaction occurs:
2PbS(s) + 3O2(g) → 2PbO(s) + 2SO2(g)
If 50.0 g of PbS reacts with 25.0 g of oxygen, how many grams of PbO will be formed?
A) 116 g B) 46.6 g C) 163 g D) 69.9 g E) 93.2 g
5. Phosphorus trichloride can be made by the reaction:
P4(s) + 6Cl2(g) → 4PCl3(l)
What is the maximum amount of phosphorus trichloride that can be formed if 10 molecules of P4 react with 36 molecules of chlorine?
A) 4 molecules D) 24 molecules
B) 6 molecules E) 46 molecules
C) 12 molecules
6. Consider the reaction:
Zn(s) + NO3-(aq) → NH3(aq) + Zn(OH)42-(aq)
When this equation is balanced in basic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
A) 3, 3 B) 2, 1 C) 6, 8 D) 5, 6 E) 2, 3
7. If the pH of a blood sample is 7.60, what is the H3O+ concentration in the blood?
A) 7.6 M B) 2.5 x 10-8 M C) 2.5 x 10-7 M D) 2.5 x 10-9 M E) 6.4 M
8. If CH3NH2 is added to water, what other compound could also be added in order to make a buffered solution?
A) H2O D) NaCH3NH2
B) NaOH E) none of these is correct
C) CH3NH3Cl
9. What is the pH of a 0.015 M NaOH solution?
A) 0.015 B) 1.82 C) 7.00 D) 12.18 E) -1.82
10. Ethane, C2H6, can be formed by reacting acetylene, C2H2, with hydrogen gas as follows:
C2H2(g) + H2(g) ↔ C2H6(g) Exothermic
What change will be observed if the temperature of the reaction mixture at equilibrium were increased?
A) The concentration of C2H6 will increase.
B) The concentration of both C2H2 and H2 will increase.
C) The concentration of both C2H2 and H2 will decrease.
D) The concentration of H2 only will decrease.
E) There will be no change in the equilibrium concentrations.
11. Consider the following reaction:
N2O4(g) ↔ 2NO2(g)q = +58.2 kJ
What will cause an increase in the concentration of NO2 at equilibrium?
A) The NO2 concentration can never change because the reaction is at equilibrium.
B) an increase in temperature
C) an increase in pressure
D) a decrease in volume
E) adding a catalyst
12. Which of the following compounds is an aldehyde?
A) CH3CH2OH D) CH3COCH3
B) CH3CH2COCH3E) CH3CHO
C) CH3OCH3
13. Which one of the following reactions is an example of an oxidation-reduction reaction?
A) BaO(s) + CO2(g) → BaCO3(s)
B) H2(g) + F2(g) → 2HF(g)
C) CaCO3(s) → CaO(s) + CO2(g)
D) HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
E) Ba2+(aq) + SO42-(aq) → BaSO4(s)
14. Consider the following reaction:
Mn(s) + CuSO4(aq) → MnSO4(aq) + Cu(s)
Which of the following statements regarding this reaction is correct?
A) Manganese is neither oxidized nor reduced.
B) The sulfate ion is oxidized.
C) Copper is the reducing agent.
D) Manganese is the oxidizing agent.
E) Each copper gains two electrons.
15. In which of the following choices is the oxidation number incorrect?
A) Cr3+(aq); oxidation number = 3+D) K+(aq); oxidation number = 1+
B) Cl-(aq); oxidation number = 1-E) Ag(s); oxidation number = 1+
C) F2(g); oxidation number = 0
16. What is the oxidation number of boron in sodium tetraborate, Na2B4O7?
A) +12 B) -3 C) +14 D) +3 E) +4
17. Consider the reaction:
H3AsO3(aq) + BiO3-(aq) → H3AsO4(aq) + Bi(s)
When this equation is balanced in acidic solution, the coefficient for water will be__________, and the number of electrons transferred will be __________.
A) 2, 6 B) 2, 1 C) 1, 10 D) 1, 2 E) 2, 3
18. Given that 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g), if 82.0 g of NH3 react with sufficient oxygen, how many grams of NO will be formed?
A) 145 g B) 5.80 x 102 g C) 46.5 g D) 186 g E) 11.6 g
19. Balance the following skeletal equation:
Ba(NO3)2(aq) + K2SO4(aq) → BaSO4(s) + KNO3(aq)
A) Ba(NO3)2(aq) + K2SO4(aq) → BaSO4(s) + KNO3(aq)
B) 2Ba(NO3)2(aq) + K2SO4(aq) → 2BaSO4(s) + KNO3(aq)
C) 2Ba(NO3)2(aq) + 2K2SO4(aq) → 2BaSO4(s) + 2KNO3(aq)
D) 2Ba(NO3)2(aq) + 2K2SO4(aq) → 2BaSO4(s) + 3KNO3(aq)
E) Ba(NO3)2(aq) + K2SO4(aq) → BaSO4(s) + 2KNO3(aq)
20. The condensed structural formula for the molecule shown in the figure is__________.
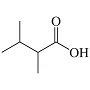
A) C6H16O2 D) (CH3)2CHCH(CH3)CO2H
B) CH3CH2CH(CH3)3CO2HE) CH3CH2CH(CH3)2CO2H
C) (CH3)2CH(CH2)2CO2H
21. Which of the following is the molecular formula for the molecule represented in the figure?
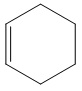
A) C6H6 B) C6H8 C) C6H10 D) C6H12 E) C6H16
22. To which class of compounds does the molecule shown in the figure belong?
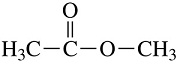
A) ketone B) aldehyde C) alcohol D) ester E) carboxylic acid
23. The position of equilibrium would not be appreciably affected by changes in the volume of the container for
A) NiO(s) + CO(g) ↔ Ni(s) + CO2(g)D) 2CO(g) + O2(g) ↔ 2CO2(g)
B) BaCO3(s) ↔ BaO(s) + CO2(g)E) PCl5(s) ↔ PCl3(g) + Cl2(g)
C) 2H2O(g) ↔ 2H2(g) + O2(g)
24. Write the equilibrium constant expression for the reaction:
Pb2+(aq) + 2Cl-(aq) ↔ PbCl2(s)
A) Keq = {[Pb2+] [Cl-]2} / [PbCl2] D) Keq = {[Pb2+] [Cl-]} / [PbCl2]
B) Keq = [Pb2+][ Cl-]2 E) Keq = [PbCl2] / {[Pb2+] [Cl-]2}
C) Keq = 1 / {[Pb2+] [Cl-]2}
25. What is the balanced chemical equation that corresponds to the equilibrium constant expression (assuming a homogeneous equilibrium in the gas state)?
Keq = {[C] [D]} / {[A] [B]2}
A) A + B2 ↔ C + D D) C + D ↔ A + B2
B) A + 2B ↔ C + D E) C + D ↔ A + 2B
C) C + D ↔ A + B
26. The graph shows the change in concentration of reactant and product as a reaction proceeds. At what point is equilibrium first reached?
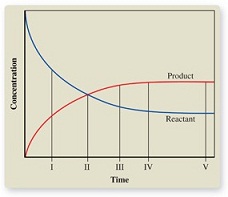
A) I B) II C) III D) IV E) V
27. According to collision theory, the increase in the rate constant with increasing temperature is due mostly to the fact that
A) the activation energy decreases with increasing temperature.
B) the fraction of the collisions having sufficient energy to react increases with increasing temperature.
C) the pressure of the reactants increases with increasing temperature.
D) the heat change for most reactions is negative.
E) the fraction of the collisions that have the proper orientation for reaction increases with increasing temperature.
28. Silver nitrate, AgNO3, can be used to test for the presence of chloride ions in solution, because it readily forms a precipitate of AgCl. What volume of 2.0 M AgNO3 will be required to react with 50.0 mL of a 0.10 M HCl solution?
AgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq)
A) 0.25 mL B) 25 mL C) 5.0 x 101 mL D) 2.5 x 102 mL E) 2.5 mL
29. What is the percent-by-mass concentration of antifreeze (ethylene glycol, C2H6O2) in an aqueous solution that contains 420.0 g of ethylene glycol in 1.00 L of solution. The density of the solution is 1.05 g/mL.
A) 2.50% B) 42.1% C) 40.0% D) 44.1% E) 60.0%
30. What mass of sodium nitrate is dissolved in 455 g of a solution that is 15.0% by mass NaNO3?
A) 68.3 g B) 30.3 g C) 4.40 x 102 g D) 3.03 x 103 g E) 15.0 g
31. Rank the following substances in order of increasing boiling point: F2, Ne, He, Cl2
A) F2 < Ne < He < Cl2 D) He < Ne < F2 < Cl2
B) F2 < He < Ne < Cl2 E) Cl2 < F2 < Ne < He
C) F2 < Cl2 < He < Ne
32. Which of the following substances can participate in hydrogen bonding?
A) CH4 B) HF C) CH3COCH3 D) SiH4 E) all of these choices are correct
33. Which choice correctly lists the intermolecular forces present in CH3NH2?
A) London forces only
B) dipole-dipole forces only
C) London forces, dipole-dipole forces, and hydrogen bonding
D) dipole-dipole forces and hydrogen bonding
E) hydrogen bonding only
34. The forces that hold CO2 together in the solid state are:
A) ionic bonds.
B) dipole-dipole forces.
C) London dispersion forces only.
D) covalent bonds.
E) attractions between nuclei and delocalized valence electrons.
35. Calculate the molar mass of a gas that has a density of 1.428 g/L at STP.
A) not enough information D) 32.01 g/mol
B) 14.28 g/mol E) 0.7002 g/mol
C) 0.4460 g/mol
36. A sample of H2 is collected over water at 22°C. If the total pressure of the sample is 744 torr, what is the partial pressure of the H2? The vapor pressure of water at 22°C is 19.8 torr.
A) 37.6 torr B) 0.979 atm C) 764 torr D) 724 torr E) 744 torr
37. If a 7.00 L container is filled with O2 to a pressure of 995 torr at 33.0°C, calculate the mass of the oxygen in the container.
A) 11.7 g B) 0.365 g C) 277 g D) 2.57 x 103 g E) 0.0854 g
38. Which of the following gases will have a density of 2.104 g/L at 303 K and 1.31 atm?
A) He B) Ne C) Ar D) Kr E) Xe
39. Given a fixed amount of gas held at constant pressure, calculate the volume it would occupy if a 3.50 L sample were cooled from 90.0oC to 30.0oC.
A) 1.17 L B) 10.5 L C) 4.19 L D) 2.92 L E) 1.75 L
40. What volume of H2 would be collected at 21.5oC and a pressure of 695 torr if 5.25 g of zinc react according to the equation:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
A) 1.06 L B) 4.25 L C) 1.80 L D) 2.12 L E) 5.49 L
41. Arrange the following bonds in order of increasing polarity: Cl-S, Cl-P, Cl-Si, Cl-Cl
A) Cl-S < Cl-P < Cl-Si < Cl-Cl D) Cl-Cl < Cl-S < Cl-P < Cl-Si
B) Cl-S < Cl-Si < Cl-P < Cl-Cl E) Cl-Cl < Cl-P < Cl-Si < Cl-S
C) Cl-Si < Cl-S < Cl-Cl < Cl-P
42. Predict the molecular shape and give the approximate bond angles of the SiH4 molecule.
A) linear, 180° D) trigonal pyramidal, 109.5°
B) trigonal planar, 120° E) bent, 120°
C) tetrahedral, 109.5°
43. Which of the following molecules is polar?
A) BF3 B) CH4 C) CS2 D) PCl3 E) BeCl2
44. The electron configuration 1s22s22p6 applies to all of the following species except:
A) Ne B) F- C) O2- D) Na+ E) Ca2+
45. Rank the following elements in order of increasing atomic size: Al, Ba, O, C
A) Al < Ba < O < C D) O < C < Al < Ba
B) Ba < Al < O < C E) C < O < Al < Ba
C) Ba < Al < C < O
46. Some elements have electron configurations that deviate from normal electron filling rules. Which element has the ground-state electron configuration [Ar]4s13d10?
A) Ni B) Ag C) Cd D) Sn E) Cu
47. The element that has four completely filled s sublevels, and three d electrons is:
A) V B) Cr C) Nb D) Ti E) Sc