Reference no: EM131067246
1. Give three examples of particulate matter found in air. Explain the difference between PM2.5 and P1\410 in terms of size and health effects.
2. Radon is one of the noble gases found in Group 8A on the periodic table. Which properties does it share with the other inert gases? In which way is it distinctly different?
3. a. The concentration of argon in air is approximately 0.9%. Express this value in ppm.
b. The air exhaled from the lungs of a smoker has a concentration of 20-50 ppm CO. In contrast, air exhaled by nonsmokers is 0-2 ppm CO. Express each concentration as a percent.
c. In a tropical rain forest, the water vapor concentration may reach 50,000 ppm. Express this as a percent.
d. In the dry polar regions, water vapor may be a mere 10 ppm. Express this as a percent.
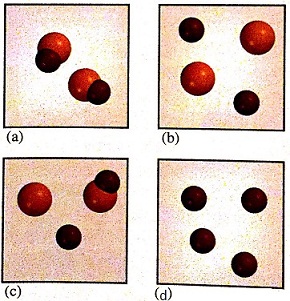
4. In these diagrams, two different types of atoms are represented by color and size. Characterize each sample as an element, a compound, or a mixture. Explain your reasoning.
5. Express each of these numbers in scientific notation.
a. 1500 m, the distance of a foot race
b. 0.0000000000958 m, the distance between O and H atoms in a water molecule
c. 0.0000075 m, the diameter of a red blood cell
6. Consider this portion of the periodic table and the groups shaded on it.
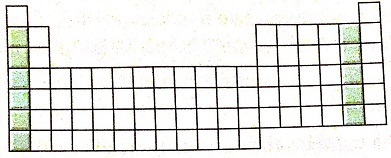
a. What is the group number for each shaded region?
b. Name the elements that make up each group.
c. Give a general characteristic of the elements in each of these groups.
7. Classify each of these substances as an element, a compound, or a mixture.
a. a sample of "laughing gas" (dinitrogen monoxide, also called nitrous oxide)
b. steam coming from a pan of boiling water
c. a bar of deodorant soap
d. a sample of copper
e. a cup of mayonnaise
f. the helium filling a balloon
8. Balance these equations in which ethane (C2H4) burns in oxygen.
a. C2H4(g) + O2(g) → C(s) + H2O(g)
b. C2H4(g) + O2(g) → CO(g) + H2O(g)
c. C2H4(g) + O2(g) → CO2(g) + H2O(g)
9. Count the atoms on both sides of the arrow to demonstrate that these equations are balanced.
a. 2C3H8 (g) + 7O2 (g) → CO(g) + 8 H2O (l)
b. 2C8H18 (g) + 25O2 (g) → 16 CO2 (g) + 18 H2O (/)
|
The phone computer the incemental net income
: Signal mistakenly porduced 1,425 defective cell phone. The cost $68 each to produce A salvege company will but the defective phone are they are for $34 eazh.
|
|
Expansion from an ethical perspective
: In 3-5 paragraphs, (1) evaluate the possibility of this line expansion from an ethical perspective, and (2) considering what you have learned about groups and the family, make a decision for the company. Be sure to explain your position and descr..
|
|
Utilize the advertising objectives
: Assume you have narrowed down the target market to middle to upper class women aged 18-25 living in major cities. Explain your reasoning for each and utilize the advertising objectives to help convey your message.
|
|
Future of the new product
: Find a product that you have noticed recently and perceive to be new. Examine how the internet is being used to bring this new product to the consuming public. In 3-5 paragraphs, answer the following questions: What do you perceive the future of th..
|
|
What is the group number for each shaded region
: What is the group number for each shaded region? Classify each of these substances as an element, a compound, or a mixture. Give a general characteristic of the elements in each of these groups.
|
|
Income tax purposes as personal use property
: Tina owns a condominium in Santa Barbara. During the current year, she incurs the following expenses related to the property:
|
|
What do the community members spend their money
: what do the community members spend their money
|
|
Definition of marketing offered in the course content
: Creating Value for Customers. Consider the customers you believe currently use your product or service and the definition of marketing offered in the course content. As we begin our study of marketing, what are your preliminary thoughts as to how ..
|
|
Difference in patient bmi based on alcohol consumption
: Is there a difference in patient BMI following the "Eat Your Heart Out" education intervention and difference in patient BMI based on alcohol consumption?
|