Reference no: EM131025390
The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2.
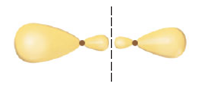
(a) Which valence atomic orbitals of I and of Br are used to construct the MOs of IBr?
(b) What is the bond order of the IBr molecule?
(c) One of the valence MOs of IBr is sketched here. Why are the atomic orbital contributions to this MO different in size?
(d)What is the label for the MO?
(e) For the IBr molecule, how many electrons occupy the MO?
Which would you expect to take up more space, a P-F bond or a P-Cl bond? Explain.
(c) Predict the molecular geometry of PF4Cl.How did your answer for part (b) influence your answer here in part (c)?
(d) Would you expect the molecule to distort from its ideal electron-domain geometry? If so, how would it distort?
|
Identify nursing leadership priorities
: Identify Nursing Leadership Priorities. The goal of your course project is to showcase your understanding of systems, organizations, and leadership and demonstrate your ability.
|
|
Prepare the appropriate entries for both the lessee
: Twelve annual payments of $58,000 (including executory costs) beginning January 1, 2013, the inception of the lease and each December 31 thereafter through 2021.
|
|
What is the physical basis for the vsepr model
: What is the physical basis for the VSEPR model? When applying the VSEPR model, we count a double or triple bond as a single electron domain.Why is this justified?
|
|
Peruse professional organization and government web sites
: Peruse professional organization and government Web sites for ideas, themes, and current information relating to systems, organizational, and nursing leadership with which to address your issues of priority for the course project.
|
|
What is the bond order of the ibr molecule
: The iodine bromide molecule, IBr, is an interhalogen compound. Assume that the molecular orbitals of IBr are analogous to the homonuclear diatomic molecule F2. Which valence atomic orbitals of I and of Br are used to construct the MOs of IBr?
|
|
How many electrons occupy the mo in the figure
: Consider the molecular orbitals of the P2 molecule. Assume that the MOs of diatomics from the third row of the periodic table are analogous to those from the second row. Which valence atomic orbitals of P are used to construct the MOs of P2?
|
|
What is national saving
: Given the choices of world real interest rate in the above graph, the world real interest rate that most likely occurs when this country runs a trade surplus
|
|
What will happen to the level of excess reserves in system
: Assume that the banking system has $200 billion in reserves. There are no excess reserves in the system. If the reserve requirement is decreased from 10 percent to 8 percent, what will happen to the level of excess reserves in the system
|
|
Water is the most importnt substance on earth
: Can the model supply heat/temp. data in other ranges, for example at -5 C or at 150 C? Explain why or why not.
|