Reference no: EM131128473
1.
a) What is meant by the assumption of irreversibility in the context of binding reactions?
b) Describe the differences between the "initial rate" method and "relaxation methods" to obtain rate constants.
c) The observed rate constant for the formation of a complex is larger when the experiment is performed under conditions when the reaction must be treated as reversible rather than irreversible. Explain why this is the case.
2) In a buffered solution, the concentration of free protons is constant. Any protons that are either taken up or released during a biochemical process must come from or end up on the buffer molecules. This applies to biochemical reactions as well as ligand binding. When protons are released upon ligand binding to a protein, the protons can be considered to bind to the buffer. If protons are taken up from solution upon ligand binding, they are taken from protonated buffer molecules. As a result, if binding is monitored by ITC, the heat measured must also include the heat of ionization of the buffer if it loses or picks up protons during complex formation. An example of this is described in slides 44 and 45 of lecture 19, which we did not go through in class. Look these over before doing the following problem.
The following ITC data were obtained in studying the binding of the estrogen receptor α to duplex DNA. It was found that the observed heat of the reaction depended on the buffer in which the experiment was performed.
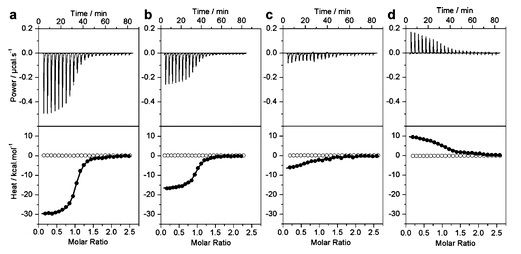
a) Phosphate; b) Hepes; c) Tricine; d) Tris buffers
The buffers used have different enthalpies of ionization (?Hoion)
Phosphate: 1.22 kcal/mol
Hepes: 5.02 kcal/mol
Tricine: 7.64 kcal/mol
Tris: 11.35 kcal/mol
a) In the presence of phosphate buffer, is the binding of DNA to the protein endothermic or exothermic? What is your reasoning?
b) When Tris is deprotonated (ionization), is heat released or taken up from the surroundings?
c) Are protons taken up or released upon binding of the protein and DNA, or is there no change in protonation upon forming the complex. Explain.
|
How will that affect total inventory costs
: The Olde Town Microbrewery makes Townside beer, which it bottles and sells in its adjoining restaurant and by the case. It costs $1,700 to set up, brew, and bottle a batch of the beer. The annual cost to store the beer in inventory is $1.25 per bottl..
|
|
By how much must an enzyme reduce the activation energy
: By how much must an enzyme reduce the activation energy of a reaction at 37°C in order for the reaction to occur 100 times faster than in the absence of the enzyme?
|
|
Use free advertising as they define public relations
: Many companies with small budgets mistakenly think they can use "free" advertising as they define public relations. Indeed, some companies do rely only on public relations only if the unique selling proposition is so newsworthy that the news media wi..
|
|
Non-profit organization to establish human resources plan
: Imagine you are a consultant working with a start-up non-profit organization to establish their Human Resources plan. What does the plan look like and why? What are some challenges in the current workforce that you are likely to face, and should cons..
|
|
What is meant by the assumption of irreversibility
: What is meant by the assumption of irreversibility in the context of binding reactions?- Describe the differences between the "initial rate" method and "relaxation methods" to obtain rate constants.
|
|
What is the safety stock and the average inventory
: A company stocks an SKU with a weekly demand of 225 units and a lead time of 3 weeks. Management will tolerate one stockout per year. If sigma for the lead time is 175 and the order quantity is 800 units, what is the safety stock, the average invento..
|
|
To use the purchase method of inventory accoutning
: Which requirement apply to proprietary find but not the government fun? Report circulating assets use consumption method inventory accounting To report current liability To use the purchase method of inventory accoutning
|
|
Biological-functional conflict and symbolic interaction
: Sociologists explain deviance by three (3) major perspectives: biological, functional conflict, and symbolic interaction. Identify your role, for example, as a parent and which perspective best reflects your personal experience. Discuss the main reas..
|
|
Provide your maximum design values in a tabulated form
: Considering the above combinations and using Spacegass determine the maximum (positive and negative) reactions, shear forces and bending moments the beam should be designed for. These maximum values are known as "design" values.
|