Reference no: EM131685545
Assignment: Steam-Water Double Pipe Heat Exchanger Experiment
Equipment
There are two systems in this experimental unit shown in the schematic diagram below. System 1 uses condensing steam in the shell side to heat hot tap water in the tube side in a co-current flow configuration. System 2 uses cold tap water to cool the heated water on the tube side (coming from System 1). System 2 may be (and should be) run in both the co-current and the counter-current flow patterns. Both double pipe heat exchangers are identical and contain the same heat transfer area. Both are insulated.
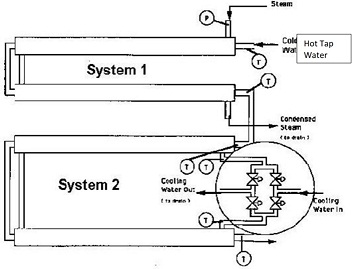
Questions:
I. Give examples of two other types of heat exchangers aside from the double pipe heat exchanger. Explain how the other heat exchangers work and comment on their efficiencies compared to that of the double pipe heat exchanger.
II. What is "heat duty"? What is its definition? How will you calculate heat duty values in this experiment for System I and System II?
III. Draw a process flow diagram for the heat exchangers. Mark clearly the counter- and co-flow double pipe heat exchangers. Include both material and energy fluxes. Discuss co- vs. counter-current heat exchangers, including differences in heat transfer driving forces and efficiencies.
IV. What is the "overall heat transfer coefficient"? What is its unit? How will you calculate it from experimental data? What is the log mean temperature difference (LMTD) and how is it different than temperature difference? How is LMTD used and how will it be calculated?
V. How will you calculate predicted heat transfer coefficient? If the predicted heat transfer coefficient differs from that determined from experimental data, what might account for their differences?
VI. What is "heat transfer resistance"? Give examples of a "low heat transfer resistance" and a "high heat transfer resistance" and rationalize their differences.
VII. What are the sources of uncertainties in this experiment? What category, random error or systematic error, does each of the uncertainty fall into? Describe a general strategy by which you will use to propagate these uncertainties in your calculations.