Reference no: EM131046878
1. This energy diagram is for the thermal decomposition of solid mercury (II) oxide (also known as mercuric oxide) into liquid mercury and oxygen gas.
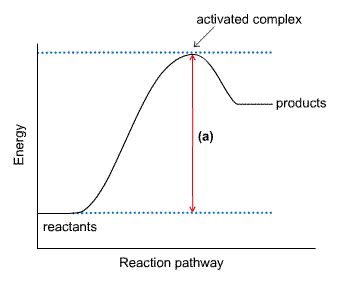
• Write a balanced equation for the reaction.
• Explain what feature is shown by the arrow labeled (a).
• Using chemical symbols and dashed lines (this can be done with type), draw what the activated complex or transition state might look like.
• Is this reaction exothermic or endothermic? Explain.
2. The diagram below shows steps in the exothermic chemical reaction of bromomethane with hydroxide to form methanol and bromide ion. The black spheres are carbon atoms, the white spheres are hydrogen atoms, the red spheres are oxygen atoms, and the blue dots are bromine atoms.
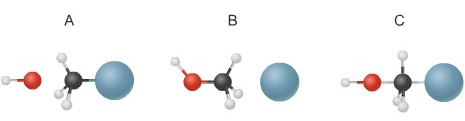
• Write a balanced chemical equation to represent the reaction.
• Using the figures in the diagram, draw the steps of the reaction in the correct order, label each molecule in the drawings, and describe what is happening in the reaction.
• Draw an energy diagram of the reaction in which you label the energies of each step in the reaction and the activation energy.
3. This graph shows the rates of reaction in a chemical reaction with and without the addition of an enzyme.
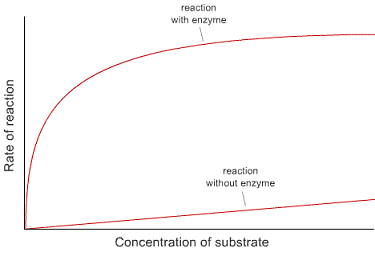
• Explain the shape of each line. What is happening to the reaction rate as the reactant concentration
is changed?
• Explain what the enzyme appears to be doing.
• Why does the curve of the reaction with enzyme flatten out?
4. Draw an energy diagram for an endothermic reaction without a catalyst (use a solid line) and with a catalyst (use a dotted line). Label all parts of the diagram. Explain what a catalyst is and how a catalyst influences the rate of a reaction.
Part 2
1. You watch water falling from the top of a waterfall to the bottom. How would you characterize this physical phenomenon in terms of spontaneity? What if you wanted to move the water from the bottom of the falls to the top-how would you characterize that reaction?
2. Consider the following reaction occurring in a closed chemical system. Assume that this reaction is at equilibrium and that in general the reaction to the right is favored.
CH3CH2OH + 3O2 ↔ 2CO2 + 3H2O ?H = -1,235 kJ/mol
• What type of chemical reaction is this?
• If more CH3CH2OH is added to the system, how will the reactions shift to reach equilibrium again?
• If water is extracted from the system, how will the reactions shift to reach equilibrium again?
• If heat is removed from the system, how will the reactions shift to reach equilibrium again?
• What is the name of the principle that helps you predict each of these shifts in equilibrium?
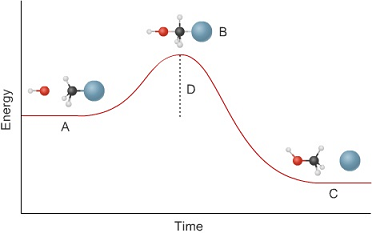
3. This graph shows the energy involved in a reaction involving two molecules. Describe what is happening or what the state of the reaction is at A, B, C, and D. Then tell if this is an exothermic or an endothermic reaction.
4. Chalcocite is an ore from which copper can be extracted for commercial use. The formula for this important ore is Cu2S. In the laboratory it is formed by the following reaction:
2Cu + S → Cu2S
The reaction takes place in laboratory conditions that allow the reactant atoms to move freely about in the reaction vessel. Explain this reaction in terms of collision theory.
Part 3
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
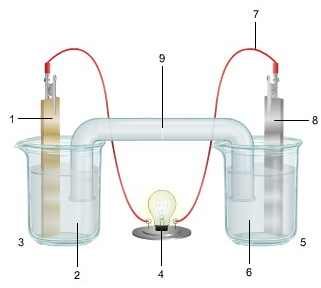
In your response, do the following:
• Label all parts (1-9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
2. The diagram shows a Zn/MnO2-KOH alkaline dry cell.
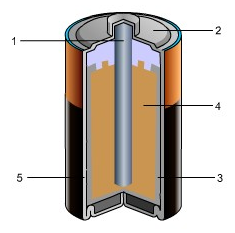
In your response, do the following:
• Label all parts.
• Label the cathode and the anode, including the charges on each.
• Show the flow of electrons.
• Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
3. Compare and contrast a dry cell with a voltaic cell by filling in the table.
4. The diagram shows an electrochemical cell with a gold strip (left) and aluminum glasses (right).
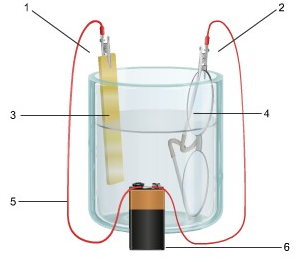
In your response, do the following:
• Label all parts.
• Label the cathode and the anode, including the charge on each.
• Show the flow of electrons.
• Describe what type of electrochemical cell is pictured and what its use is.
• Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
Part 4
1. Draw butane (C4H10). What class of organic molecule is butane?
Describe an isomer. Draw an isomer of butane.
2. Draw the three-carbon hydrocarbons propane, propene, and propyne. Classify each molecule, and explain the differences between them.
3. Draw a general diagram of a triglyceride. Describe the various types of biologically important lipids, their general structures, and their functions.
Part 5
1. Compare and contrast the processes of nuclear fission and fusion, showing similarities and differences. Describe which, if any, of these processes is naturally occurring. If so, where?
2. The data in the table show the amount of a 500 g sample of sodium-24 over time.
|
Time (h)
|
Na-24 (g)
|
|
Time (h)
|
Na-24 (g)
|
|
0
|
500
|
55
|
39
|
|
5
|
397
|
60
|
31
|
|
10
|
315
|
65
|
25
|
|
15
|
250
|
70
|
20
|
|
20
|
196
|
75
|
16
|
|
25
|
158
|
80
|
12
|
|
30
|
125
|
85
|
10
|
|
35
|
99
|
90
|
8
|
|
40
|
79
|
95
|
6
|
|
45
|
63
|
100
|
5
|
|
50
|
50
|
|
Make a graph of the data (remember to label all axes and title the graph).
What is the half-life of sodium-24? Explain how you determined your answer.
Sodium-24 decays by beta emission. Write a nuclear equation that shows the decay of sodium-24 and
its products.
3. Describe the subatomic structure of the nucleus, including the structure of each nucleon. Draw a picture.
Describe the forces that hold the nucleus together and draw them on your diagram.
Explain how beta emission works.
4. Describe what happens in a fission reaction.
What is a commonly used isotope in a nuclear fission reactor?
Describe how a fission nuclear reactor power plant works.