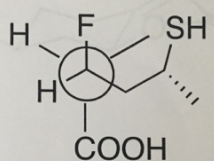
Reference no: EM13773443
Question 1:
a. Please convert the following Newman Projection into a wedge-dash 2D structure.
b. Label all stereocenters R or S,
c. Label all functional groups.
d. Draw out the other two staggered Newman Projections for this molecule.
Indicate which of the three staggered structures is the most stable and give reasons for your choice.
Question 2:
a. Please convert the following cyclohexane conformation into a wedge das 2D structure.
b. Label all star centers R or S.
c. Label all functional groups.
d. Can this molecule rig flip? Please explain whyiwhy not,

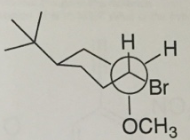
Question 3:
a. Please convert the following Newman Projection into a wedge-dash 2D structure.
b. Label all stereocenters R or S.
c. Label all functional groups,
d. Convert the wedge-dash 2D structure into a 3D cyclohexane structure.
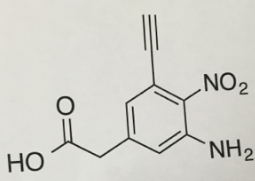
Question 4:
What are the three most acidic ptcrtons in molecule, Please Label them on the structure and rank them ft, #2, 03. Please provide tholokigh reasoning (and drawings!) to support your choices and racking.
b What is the most basic atom in this molecule? Please provide reasoning as to why
c. Please label the functional groups in this molecule.
d, Vkihat is the hybridization of N iii NO2? What is the hybridization pi N in N Hz?