Reference no: EM13980965
Please can you show a simplified working of your answers and simplified working of your calculations
Section -1:
Answer ALL parts of this question
(a) Assign the protons in the molecules 1 and 2 below to their appropriate 1H NMR spin systems using the simplified Pople convention.
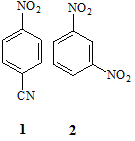
(b) Predict and sketch the precise appearance of the 1H NMR spectrum (assume 1st order appearance) of compound 2 in part (a) above. Your answer should include an illustrative splitting tree diagram to establish the appearance of one of the signals, as well as all anticipated chemical shifts derived from NMR parameter tables. A full answer will include a consideration of long-range splitting effects.
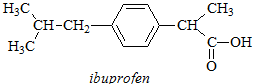
(c) A sample taken from the scene of a suspected suicide is believed to contain ibuprofen and a solution of it in D2O is prepared and subjected to 1H NMR analysis. By reference to its chemical structure below identify the intensity, multiplicity and approximate chemical shift of each signal expected in the 1H NMR spectrum of the sample. You may use NMR parameters tables provided to assist you in your estimation of chemical shifts but you do NOT need to illustrate or explain the use you have made of parameter tables in your written answer, nor do you have to explain the splitting patterns you predict.
Answer ALL parts of this question
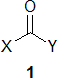
(a) Acyclic ketones such as 1 (where X and Y indicate alkyl groups) commonly undergo α- cleavage when subjected to electron impact mass spectrometry (EI-MS). Give the mechanism for α-cleavage and predict the identity of the molecule that gives signals at m/z values of 100, 71 and 57 amu during EI-MS, explaining your reasoning.
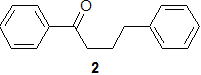
(b) The molecule 2 shown may undergo a McLafferty rearrangement. Draw a mechanism to show how this process operates for this molecule, and identifying the fragments that result, indicating which would be observed by an (EI) mass spectrometer.
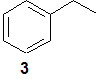
(c) Predict the major fragment ions that might be observed for compound 3.
Section 2 - you must attempt at least one question from this section
1. Answer ALL parts of this question.
(a) Asymmetry factor (As) is used to determine the peak shape of compounds separated by chromatography. Indicate the As values for the following peak shapes:
i) fronting
ii) Gaussian (symmetrical)
iii) tailing
(b) Solid phase extraction (SPE) is commonly used for aqueous sample preparation prior to chromatographic analyses. Outline the process of operation of SPE with reversed phase sorbent.
(c) What are the potential problems associated with split/splitless injection for gas chromatography (GC)?
(d) Why is it necessary to use a temperature programme in GC analysis of a complex mixture? Include in your answer a sketch of the chromatograms you would expect from the analysis under isothermal and temperature-programmed conditions.
(e) Gas chromatography coupled to a mass spectrometer (GC-MS) provides an effective tool for analysis. Explain ONE method of ionisation commonly used for GC-MS.
2. Answer ALL parts of this question.
(a) Two compounds were separated by HPLC using a 25-cm long column. The following information was obtained from the chromatogram via the computer output and the dead time was 1.68 min.
|
Peak number
|
Retention time (min)
|
Peak height
(mV)
|
Peak area (mV.min)
|
|
1
|
4.87
|
41120
|
54641
|
|
2
|
5.27
|
39122
|
45687
|
Calculate:
i) the separation factor (α) between these two peaks.
ii) the number of theoretical plates or column efficiency (N) for each peak.
iii) the height equivalent to a theoretical plate (H) for each peak
(b) Reversed phase and normal phase - high performance liquid chromatography (HPLC) are used to separate organic compounds of particular polarity. Compare the two methods with respect to stationary phase polarity, mobile phase polarity, and order of analyte elution.
(c) Examine the reasons for surface deactivation of silica-based C18 column in reversed phase - HPLC.
(d) What are the advantages and disadvantages of the ‘selected ion monitoring' mode for known compounds relative to ‘full scan' mode for data acquisition in HPLC-MS?
|
To reduce the costs of resolve insurance disputes
: To reduce the costs of resolve insurance disputes, insurers have required that customers use arbitration. Arbitrators are required to be knowledgeable about medicine and insurance contacts. Why might you anticipate that the arbitration mechanism woul..
|
|
Explain the definition and concept of passionate
: Explain the definition and concept of passionate and companionate love and compare and contrast both types of love. In your opinion, is there more than just passionate and companionate love
|
|
Choose two of theories above to be the central piece.
: Theories are: White privilege, structural racism, Race as social construct, the racial contract, racial moral cognitive dysfunction/double standards of morality and empathy based on race, epistemology of forgetfulness...
|
|
Typical managers can meet half of these milestones
: A convenience store manager earns a base salary plus small bonuses for each of ten different possible monthly milestones he meets. Typical managers can meet half of these milestones. Do they miss the others by a little or a lot?
|
|
What are the potential problems associated with split
: What are the potential problems associated with split/splitless injection for gas chromatography (GC) and why is it necessary to use a temperature programme in GC analysis of a complex mixture?
|
|
Why does the observer see a bright circle on the surface
: A lamp emitting red light is placed at the bottom of a tank of liquid with depth H = 16.0 cm and index of refraction n = 1.38 (for red light). Above the liquid is air, which has an index of refraction of 1.00. On the surface of the liquid above th..
|
|
A firm in a monopolistically competitive market faces
: A firm in a monopolistically competitive market faces a- downward sloping demand curve because the firm's product is different from those offered by other firms. downward-sloping demand curve because there are only a few firms in the market.
|
|
Calculate the common financial ratios
: Using only annual numbers, please calculate the common financial ratios found in table 3.5 in the textbook for these companies(Look at the attached slide# 11). What do these ratios mean? Are the ratios similar for all of the companies?
|
|
Cause rapid fluctuations in short-run exchange rates
: Supply-side policy is based on the assumption that people's economic behavior is not affected by taxes. Interest rate differentials can cause rapid fluctuations in short-run exchange rates. In general, speculators tend to make a floating exchange rat..
|