Reference no: EM131204506
CHEMICAL BONDING
MCQ Questions
Isostructural species are those which have the same shape and hybridization. Among the given species identify the isostructural pairs.
[NF3 and BF3]
[BF4- and NH4+]
[BCl3 and BrCl3]
[NH3 and NO3-]
Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
CO2
HI
H2O
SO2
3. The types of hybrid orbitals of nitrogen in NO2+ , NO3- and NH4+ respectively are expected to be.
i) sp, sp3 and sp2
ii) sp, sp2 and sp3
iii) sp2, sp and sp3
iv) sp2, sp3 and sp
4. Hydrogen bonds are formed in many compounds e.g. H2O, HF ,NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is:
i) HF> H2O>NH3
ii)H2O >HF> NH3
iii) NH3>HF>H2O
iv) NH3 >H2O >HF
5. In PO43- ion the formal charge on the oxygen atom of P-O bond is
i) +1
ii) -1
iii) -0.75
iv) 0.75
6. In NO3- ion, the number of bond pairs and the lone pairs of electrons on nitrogen atom are
i) BH4-
ii) NH2-
iii) CO32-
iv) H3O+
7. Which of the following species has tetrahedral geometry?
i) BH4-
ii) NH2-
iii) CO32-
iv) H3O+
8. Number of π bonds and σ bonds in the following structure is :
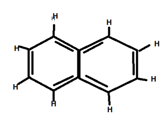
i) 6, 19
ii) 4 , 20
iii) 5, 19
iv) 5, 20
9. Which molecule or ion out of the following does not contain unpaired electrons?
i) N2+
ii)O2
iii) O22-
iv) B2
10. In which of the following molecule or ion all the bonds are not equal?
i) XeF4
ii)BF4-
iii) C2H4
iv) SiF4
|
Size of the payload in the first fragment
: In an IPv4 packet, the size of the payload in the first fragment, in octets, is equal to Total Length - (4 × IHL). If this value is less than the required minimum (8 octets for TCP), then this fragment and the entire packet are rejected. Suggest a..
|
|
Subsequent packet having a non-zero offset
: The second packet would be passed through most filter implementations because it does not have a zero fragment offset. Suggest a method that could be used by a packet filter to counter this attack.
|
|
How do the above marketing efforts influence people
: Gladwell argues that marketing research techniques like focus groups are not effective because we usually react to products quickly and without much conscious thought. So, it's better to solicit consumers' first impressions rather than getting the..
|
|
Process and ways to handle problems
: As part of the training process, your manager would like to evaluate how effective you are at this task, and asked that you complete a project: creating a three-part manual that shows a user how to build a computer, using a list of specifications...
|
|
Isostructural species are those which have the same shape
: Isostructural species are those which have the same shape and hybridization. Among the given species identify the isostructural pairs. Hydrogen bonds are formed in many compounds e.g. H2O, HF ,NH3. The boiling point of such compounds depends to a lar..
|
|
How does behavior-blocking software work
: How does behavior-blocking software work? In general terms, how does a worm propagate?
|
|
Rewrite and compose a contemporary version of everyman story
: Rewrite and compose a contemporary version of the Everyman story/morality play in one of the following forms - a children's story book, a fairy tale, or a modern short story.
|
|
Role of compression in the operation of a virus
: What is the role of compression in the operation of a virus? What is the role of encryption in the operation of a virus?
|
|
What is meant by the term development inneo-classic economic
: What is meant by the term "development" inneo-classic economics? How might your answer to this question be distinct if using a heterodox approach? Explain
|