Reference no: EM131094138
Isoleucine is degraded to propionyl-CoA and acetyl-CoA in a six-step pathway that uses chemical strategies analogous to those in the TCA cycle and the b-oxidation of fatty acids. The (out of sequence) structures of the pathway intermediates are shown below.
a)Show the sequence of reactions starting with Ile and ending with the pathway products, arranging the intermediates in the correct order.
b) Name each reaction type (for example dehydration is a type of reaction), and specify any necessary cofactors for each of the reactions
2. a) Mammalian asparagine synthetase is a glutamine-dependent amidotransferase. Efforts to identify an effective inhibitor of the human version of this enzyme for use in chemotherapy for patients with leukemia has focused on the C-terminal synthetase active site as opposed to the N-terminal glutaminase domain. Explain why the glutaminase domain is not a promising target for a useful drug.
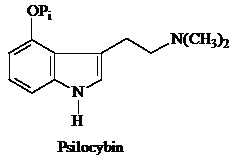
b)Psilocybin (structure shown below) is a hallucinogenic compound isolated form the fruiting bodies of the mushroom Psilocybe mexicana. Propose a pathway for its biosynthesis from an aromatic amino acid.
3. Threonine synthase (a PLP dependent enzyme) catalyzes the conversion of phosphohomoserine (structure shown below) to threonine. Write down the mechanism for this transformation.
4. Show the locations of 14C in newly synthesized UMP when the source of the isotope is uniformly labeled [14C] succinate (i.e. succinate in which every carbon is labeled), by outlining the pathway by which the label is incorporated into the product.
5.a)The molecular defect in the condition known as von Gierke's
glycogen storage disease is a deficiency in glucose-6-phosphatase. Patients suffering from this disease frequently exhibit symptoms of gout. Explain the mechanistic connection between the enzyme deficiency noted above and the appearance of such symptoms. (hint: Consider the metabolic fates of G6P.)
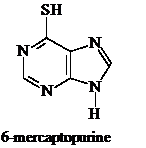
b) 6-Mercaptopurine is converted into the corresponding nucleotide through salvage pathways and acts as a potent competitive inhibitor in reactions utilizing IMP as a substrate. As a consequence, it is a clinically useful anticancer agent. The chemotherapeutic effectiveness of 6-mercaptopurine is enhanced when it is administered along with allopurinol. Explain the mechanism by which allopurinol increases the effectiveness of the anticancer agent.
6. A classic way to isolate thymidylate synthase-negative mutants of bacteria is to treat a growing culture with thymidine and trimethoprim (a dihydrofolate reductase inhibitor). Most of the cells are killed, and those that remain are nearly all the mutant-type described above.
Explain the biochemical rationale for the selection procedure by specifying the role of each substance added and the reason for the demise of non-mutant cells.
|
Decision analysis case study
: Valley of the Sun Academy (VSA) is an online school specializing in GED programs for the Phoenix area. Valley of the Sun Academy enrolls 813 students and has a part-time faculty pool of 65 online instructors.
|
|
Develop a professional profile of research interests
: Develop a professional profile of research interests that lists the scientific and humanistic creativities that most interest you.
|
|
Population mean adult sodium level
: It is known that the population standard deviation of adult sodium levels is 14 mEq. Can we conclude, at the 0.1 level of significance, that the population mean adult sodium level differs from that claimed by the laboratory?
|
|
Pair of graphs to show an perfectly competitive industry
: Create a pair of graphs to show an perfectly competitive industry and firm in long-run equilibrium. Put the graphs side by side and use the same vertical scale for both graphs.
|
|
Isoleucine is degraded to propionyl
: Isoleucine is degraded to propionyl-CoA and acetyl-CoA in a six-step pathway that uses chemical strategies analogous to those in the TCA cycle and the b-oxidation of fatty acids. The (out of sequence) structures of the pathway intermediates are show..
|
|
Stated rate of interest on the bonds
: The semiannual, 8-year bonds of Alto Music are selling at a price of $1,010. The bonds have a maturity value of $1,000 and have an effective annual yield of 8.6 percent. What is the stated rate of interest on the bonds?
|
|
Calculate its book value at the end of year
: A machine that cost $780,000 has an estimated residual value of $60,000 and an estimated useful life of six years. The company uses straight-line depreciation. Calculate its book value at the end of year 5. (Do not round intermediate calculations...
|
|
Decision making for capital budgeting
: The executive team of SNC has completed the decision making for capital budgeting for the firm. Now the team must decide which decisions and approach were the best for the company. The executive team must create a presentation to be given to the b..
|
|
What are the implied quote
: The following rates are quoted for euro in terms of US dollar and Australian dollar in New York: $/Euro=1.0750 A$/$=1.0250 (a) What are the implied quote for $ in terms of euro and for A$ in terms of $?
|