Reference no: EM133045504
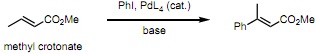
Question 1. a) Explain with a curly arrow mechanism how the Heck addition of iodobenzene to methyl crotonate works using a generalised catalyst PdL4 and why the (E)-isomer shown is formed exclusively.
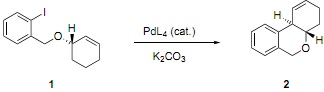
b) A Heck cyclisation of the unsaturated ether 1 gives exclusively the trans-fused isomer 2. Provide an explanation for this selectivity.
Question 2. Bromobutide is an amide-based herbicide.
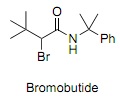
a) Design a synthesis of bromobutide using the Ritter reaction to establish the amide functionality. Draw the structures of all starting materials and reagents and include the reaction mechanism of the Ritter reaction.
b) Draw an alternative synthesis of bromobutide (no mechanisms required) and include necessary reagents.
c) Bromobutide is used as a racemate. What would be potential difficulties of synthesising one enantiomer selectively?
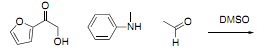
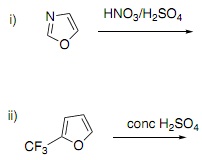
Question 3. a) Draw the products of the following two reactions and use resonance forms to explain the products.
b) Draw the products A and B of the following reaction sequence and explain your reasoning.
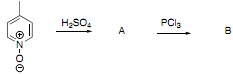
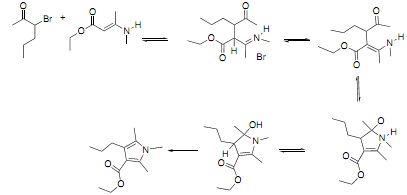
c) In the scheme below add curly arrows, relevant lone pairs and missing charges for each step and molecule / intermediate.
d) Provide the structure of the product and the curly arrow mechanism for the 3C MCR.