Reference no: EM131111193
Atoms and molecules
1. The table shows the atomic structure of six particles, represented by the letters L to Q. The particles are atoms or ions. The letters are not the symbols of the elements.
|
particle
|
electrons
|
protons
|
neutrons
|
|
L
|
6
|
6
|
6
|
|
M
|
2
|
2
|
2
|
|
N
|
12
|
12
|
12
|
|
0
|
10
|
12
|
12
|
|
P
|
6
|
6
|
8
|
|
0
|
10
|
13
|
14
|
Use the letters L to Q to answer the following questions.
(a) Which two particles are ions?
(b) Which particle is an atom of a noble gas?
(c) Which two particles are an atom and an ion of the same element?
(d) Which two particles are isotopes of the same element?
(e) Which particle has the highest atomic mass?
2. How does a magnesium atom form a bond with an oxygen atom?
A by giving one pair of electrons to the oxygen atom
B by sharing one pair of electrons, both electrons provided by the magnesium atom
C by sharing two pairs of electrons, both pairs provided by the oxygen atom
D by sharing two pairs of electrons, each atom donating one pair of electrons
3. For which of the following can graphite be used?
A as an abrasive only B as an abrasive and as an electrode
C as an electrode and as a lubricant D as a lubricant only
4. The letters X, Y and Z represent different atoms.
1940X 1939Y 2040Z
What can be deduced from the proton numbers and nucleon numbers of X, Y and Z?
A X and Y are the same element. B X and Z are the same element.
C X has more protons than Y. D Z has more neutrons than Y.
5. Which of the following contains the same number of electrons as an atom of neon?
A Cl- B Li C Li+ D O2-
6. Which gas is present in the light bulb?
A argon B krypton C nitrogen D oxygen
7. The diagram shows the structure of brass.
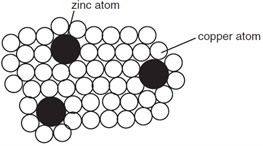
Why is brass harder than pure copper?
A The zinc atoms form strong covalent bonds with copper atoms.
B The zinc atoms prevent layers of copper atoms from slipping over each other easily.
C The zinc atoms prevent the ‘sea of electrons' from moving freely in the lattice.
D Zinc atoms have more electrons than copper atoms.
8. What is the definition of nucleon (mass) number?
A the mass in grams of an atom B the number of electrons in an atom
C the number of nuclei in a molecule D the total number of protons and neutrons in an atom
9 Which element is in Group IV, Period 5 of the Periodic Table?
A antimony B arsenic C lead D tin
10 A metal X forms oxides with the formulae XO and X2O3. Where is X in the Periodic Table?
A in Group II B in Group III C the second Period D in the transition elements
11 Fluorine is the first element in Group VII of the Periodic Table.
Which statement about fluorine is not correct?
A It is a gas at room temperature and pressure.
B Its molecules are monatomic at room temperature.
C It is a more powerful oxidising agent than chlorine.
D It forms ionic compounds with metals.
12 Graphite is used to make
A glass, B cutting tools, C electrical wiring D electrodes
13 The diagram shows the structure of sodium chloride, Na+Cl-.
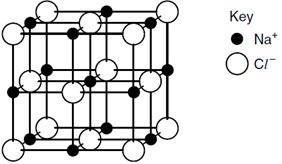
Which statement about this structure is correct?
A Each chloride ion is in contact with only one sodium ion.
B Each chloride ion is in contact with only four sodium ions.
C Each sodium ion is in contact with only four chloride ions.
D Each sodium ion is in contact with only six chloride ions.
14 Element X has an electronic structure 2.8.8.1.Element Y has an electronic structure 2.8.6.
What is made when X and Y react?
|
type of compound |
formula |
| A |
covalent compound |
X2Y |
| B |
covalent compound |
XY2 |
| C |
ionic compound |
X2Y |
| D |
ionic compound |
XY2 |
15 Which substance does not have a macromolecular structure?
A diamond B graphite C silicon dioxide D sodium chloride
16 Which atom has the same electronic configuration as the strontium ion?
A calcium B krypton C rubidium D selenium
17 Which statement about a new element, which has seven outer electrons in its atoms, is correct?
A It is monatomic. B It forms a covalent compound with hydrogen.
C It forms a positive ion. D It forms covalent compounds with Group I elements.