Reference no: EM131552752
Question: In a laboratory electrolysis experiment, 40mL of N2 gas and 60mL of O2 gas are liberated at the cathode and anode, respectively.
The room pressure and temperature are 730Torr and 25 ?C
a. Which of the following overall reactions has taken place?
2N2O → 2N2 + O2
2NO2 → N2 + 2O2
2N2O3 → 2N2 + 3O2
2N2O5 → 2N2 + 5O2
b. Figure shows a plot of the volume of N2 evolved at the cathode vs. the time in minutes. Which of the following statements most likely explains the "kink" in the graph?
i. The current was increased after 15 min.
ii. A gas leak developed above the cathode.
iii. A gas leak developed above the anode.
iv. Data points were collected more frequently after the first 15 min.
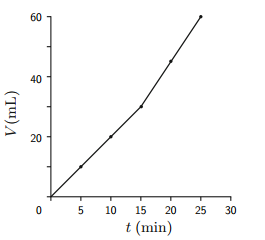
Fig. - Rate of electrolytic evolution of N2
c. At the temperature and pressure given above, what volume would 1mole of N2 gas occupy?
d. What is the total mass of N2 gas that has evolved after 25 min?