Reference no: EM132178002
Assignment: Enthalpy Changes of Reactions
Learning Outcome: Be able to relate enthalpy changes to the bonding in a range of substances.
Scenario
You are a technician in Biochem Limited, and you have been asked to produce a report comparing experimentally determined values of enthalpy changes against their theoretical values.
Tasks
Task 1 (P1)
Follow the method to determine the enthalpy changes involved in heating a known amount of water using ONLY ONE of the four alcohols provided. Calculate only the section for the alcohol you used and complete the table below with the results from the other groups.
Calculation
1. Complete the table below and use your results to work out the number of moles of the fuel burned using the formula below and show all workings and units.
Name of alcohol =
Formula of alcohol =
Molar mass of alcohol =
Mass of alcohol used =
Moles of alcohol =
(Show workings)
2. Work out the heat (q) absorbed by the water through the combustion of your alcohol; show all workings and units.
ΔT (temperature difference) of the water =
Mass of water =
q(heat absorbed) by the water =
3. Is the value positive or negative?
4. Write the equation for the standard enthalpy change of combustion for your alcohol
5. Explain the positive or negative values for your calculated enthalpy changes in terms of bond making energy being greater or less than bond breaking energy
|
Alcohol
|
Methanol
|
Ethanol
|
Propanol
|
Butanol
|
|
Volume of water placed in calorimeter (cm3)
|
|
|
|
|
|
Mass of water placed in the calorimeter (g)
|
|
|
|
|
|
Temperature of water at the start (?C)
|
|
|
|
|
|
Temperature of water at the end (?C)
|
|
|
|
|
|
Temperature difference (?C)
|
|
|
|
|
|
Mass of Spirit Burner at start (g)
|
|
|
|
|
|
Mass of spirit burner at the end (g)
|
|
|
|
|
|
Mass of fuel burned (g)
|
|
|
|
|
|
RMM of Alcohol
|
|
|
|
|
|
Moles of fuel burned (mol)
|
|
|
|
|
|
Specific heat capacity of water (Jg-1K-1)
|
4.17
|
4.17
|
4.17
|
4.17
|
|
Enthalpy of Combustion kJ/molΔHc
|
|
|
|
|
Task 2 ( P1)
Enthalpy Change of Neutralisation
1.Record the results of your experiments in the table below
|
Volume of
Sodium hydroxide added
In (cm³)
|
Temperature of
Hydrochloric acid
in (°C)
|
Temperature of
Sulphuric acid
in (°C)
|
|
5
|
|
|
|
10
|
|
|
|
15
|
|
|
|
20
|
|
|
|
25
|
|
|
|
30
|
|
|
|
35
|
|
|
|
40
|
|
|
|
45
|
|
|
|
50
|
|
|
2 Plot a graph of the results with Temperature on x-axis and Volume on y-axis.
3 Extrapolate both the ascending and descending curves, so that they cross (see Illustrations below).
4 From the point of the intersection of the curves, measure the temperature rise and the volume of alkali required for neutralisation of the acid.
5 Use the results obtained to calculate the enthalpy change in kJ/mol acid.
Graphs
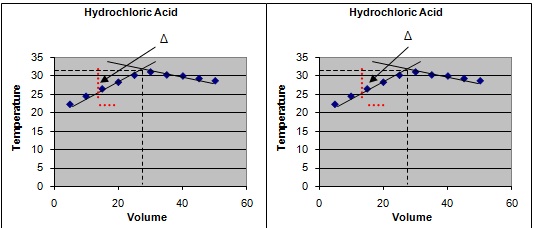
Calculation for Hydrochloric Acid
1. Calculate the energy changes of the neutralisation
(Assume the specific heat capacity of the solutions as being the same as that of the water, 4.2J/kg, and density of water as 1g/cm3)
mass of the two solutions = volume of acid + volume of alkali expressed in grams (g)
ΔT =
(temperature at intersection of the lines - lowest temperature)
C = 4.2J/kg
Enthalpy change of neutralisation (ΔHn) =
(Show workings)
= ___________________________ J
Convert to kJ
= __________________________ kJ
2. Calculate the moles of the acid used (Show workings)
moles of the acid used = _____________________________
3. Calculate the enthalpy changes for one mole of acid
(Show workings and the use accurate units)
Enthalpy changes for one mole = _____________________________
Calculation for Sulphuric Acid
4. mass of the two solutions = volume of acid + volume of alkali expressed in grams (g)
m = ________ + ______ (Show workings)
ΔT = ________________________________
(temperature at intersection of the curves- lowest temperature)
C = 4.2J/kg
Enthalpy change of neutralisation (ΔHn) =
(Show workings)
= ___________________________ J
Convert to kJ
= __________________________ kJ
5. Calculate the moles of the acid used (Show workings)
moles of the acid used = _____________________________
6. Calculate the enthalpy changes for one mole of sulphuric acid
(Show workings and the use accurate units)
Enthalpy changes for one mole = _____________________________
7. Which acid has the greatest enthalpy value _______________________
8. Write the equations for the 2 reactions
9 Relate the difference in the enthalpy changes values to the bond breaking and bond making energies involved in the neutralization reactions of the 2 acids.
Task 3 ( P1)
Enthalpy Change of Displacement and the Reactivity Series
The relative positions of metals in the reactivity series can be investigated by adding metals to solutions of salts of other metals and measuring the temperature change of the solution against time. The further apart in the series the bigger the enthalpy change will be. Complete the tables below with your experimental values and plot your results as two graphs as illustrated below. From the graphs measure a value for ΔT for each displacement
Use the values determined for ΔT to calculate the enthalpy change for each displacement
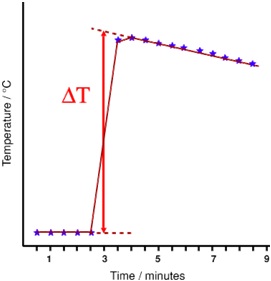
Calculation of Enthalpy Change
1. Work out the enthalpy changes made with zinc
ΔH = ________________________________________
2. Work out the moles of copper sulphate used. (Show working)
Moles of Copper Sulphate = ___________________________________
3. Work out the value of enthalpy change
ΔH =
ΔH in kJ
= _________________________________ kJ
4. Work out the enthalpy changes made with iron
ΔH = ________________________________________
5. Work out the moles of Copper Sulphate used
Moles of copper sulphate = ___________________________________
6. Work out the value of enthalpy change
ΔH =
ΔH in kJ =
= _________________________________ kJ
7. Relate the ΔH values from the two displacement reactions to the bond breaking and bond making enthalpy changes involved and to the position of zinc and iron in the reactivity series.
Task 4 (M1)
1. Using diagrams explain the solubility of sodium chloride in water and its high melting point of 801ºC in terms of its bonding and structure.
2. Water (H2O) and Hydrogen Sulphide (H2S) are both Group 6 hydrides. Use suitable diagrams to explain why water is a liquid at room temperature but Hydrogen Sulphide is a gas.
3. Hydrogen chloride gas is a covalent compound; use suitable diagrams to explain why it dissolves readily in water.
Task 5 (Unit 16 D1)
1. Use the table of electronegativity data below to analyse the type of bonding found in the following substances :
a) CsCl
b) H2O
c) Cl2
|
Element
|
Electronegativity value
|
|
Cs
|
0.8
|
|
H
|
2.1
|
|
O
|
3.5
|
|
Cl
|
3.2
|
2. Use the data from the table below to plot a graph of the boiling points of Group 7 hydrides
|
Hydride
|
Boiling point (ºC)
|
|
HF
|
20
|
|
HCl
|
- 85
|
|
HBr
|
- 67
|
|
HI
|
- 35
|
From your graph, explain the trend in boiling point in terms of the intermolecular forces found in each hydride.
3. Explain how ion size influences and the type of bonding influences the physical properties of the following:
a- Lithium fluoride is ionic but Lithium Iodide shows a lot of covalent character
b- Sodium chloride (801ºC) has a much lower melting point than magnesium oxide (2852ºC)
Attachment:- Enthalpy Changes of Reactions.rar