Reference no: EM131147314
The following volumes of a standard 10.0 ppb F solution were added to four 10.00-mLaliquots of a water sample: 0.00, 1.00, 2.00, and 3.00 mL. Precisely 5.00 mL of a solution containing an excess of the strongly absorbing AI-acid alizarin garnet R complex was added to each of the four solutions, and they were each diluted to 50.0 mL. The fluorescence intensities of the four solutions were as follows:
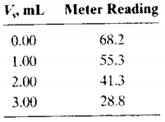
(a) Explain the chemistry of the analytical method.
(b) Construct a plot of the data.
(c) Use the fact that the fluorescence decreases with increasing amounts of the F- standard to derive a relationship like
Equation 1-3 for multiple standard additions. Use that relationship further to obtain an equation for the unknown concentration cx in terms of the slope and intercept of the standard additions plot, similar to Equcation 1-4.
(d) Use linear least squares to find the equation for the line representing the decrease in fluorescence relative to the volume of standard fluoride Vs.
(e) Calculate the standard deviation of the slope and intercept.
(f) Calculate the concentration of F- in the sample in parts per billion.
(g) Calculate the standard deviation of the result in (e).
|
Determine the optimal value of the objective function
: For the following Linear Programming model; Use graphical solution methodoloogy to find the optimal solution of the above linear programming model, wich means you need to find the optimal value of x1 and x2. determine the optimal value of the objecti..
|
|
How the large intestine works
: How the large intestine works and how one can help it to work properly
|
|
Explain the defense in depth approach
: explain the defense in depth approach and why is it important in physical security?- Which barriers would you incorporate and what factors would you use in your decision making process?
|
|
Determining the demand estimate and sales quota for product
: During the course, you will be developing a sales management plan. The project is for a fictitious product and you are the sales manager. This unit you will submit the section of the sales plan project listing the criteria for selecting the sales for..
|
|
Explain the chemistry of the analytical method
: Equation 1-3 for multiple standard additions. Use that relationship further to obtain an equation for the unknown concentration cx in terms of the slope and intercept of the standard additions plot, similar to Equcation 1-4.
|
|
How risk management strategies support equity
: How risk management strategies support equity within the workplace and Why issues of diversity within the workplace are paramount for human service workers and for management of human service organizations.
|
|
How would you describe mark zuckerbergs leadership traits
: How would you describe Mark Zuckerberg's leadership traits?- Explain your reason and refer to specific leadership traits from Theories and Leadership Approach.
|
|
Material-handling equipment in the workplace
: What do you think are two very good ways for a company to reduce the risk to their employees of injury by material-handling equipment in the workplace? Why? What is one advantage and one disadvantage of a two-handed control device.
|
|
Unpredictable action of cash drawers
: Solutions for (POS) slow printing of sales tickets and unpredictable action of cash drawers.
|