Reference no: EM13829309
Part A:
1. Complete the following Crossword Puzzle-
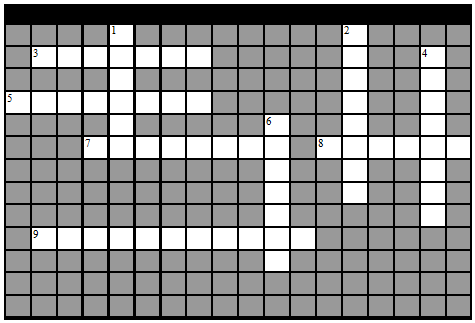
|
ACROSS
3. Away from the midline of the body
5. Close to the origin of the body part or point of attachment of a limb to the body trunk
7. Toward or at the front of the body
8. The elbow is ____ to the shoulder
9. Toward or at the body surface
|
DOWN
1. A section that divides the body into left and right
2. Toward the head end or upper part of the body
4. The heart is contained in this subcavity
6. Cavity where the brain is located
|
Part B:
Chemistry Worksheet
1. Atomic number equals the number of_____________ or ____________
Atomic mass equals the number of _____________ + _____________
Using the periodic table, fill in the chart for each Atom (Round the atomic mass to the nearest whole number).
2. Carbon (C)
Atomic # = ______Atomic Mass = _________
# of Protons = ________# of Neutrons = ________# of Electrons = ________
# of Electrons in the valence shell? _____
3. Oxygen (O)
Atomic # = ______Atomic Mass = _________
# of Protons = ________# of Neutrons = ________# of Electrons = ________
# of Electrons in the valence shell? _____
4. Sodium (Na)
Atomic # = ______Atomic Mass = _________
# of Protons = ________# of Neutrons = ________# of Electrons = ________
# of Electrons in the valence shell? _____
5. Chlorine (Cl)
Atomic # = ______Atomic Mass = _________
# of Protons = ________# of Neutrons = ________# of Electrons = ________
# of Electrons in the valence shell? _____
6. Hydrogen (H)
Atomic # = ______Atomic Mass = _________
# of Protons = ________# of Neutrons = ________# of Electrons = ________
# of Electrons in the valence shell? _____
7. Nitrogen (N)
Atomic # = ______Atomic Mass = _________
# of Protons = ______# of Neutrons = ______# of Electrons = ______
# of Electrons in the valence shell? _____
8. Identify the following as synthesis, decomposition or exchange reaction
-
- 2HG + O2 = 2HgO ____________________
-
- Fe2+ + CuSO4 = FeSO4 + Cu2+____________________
-
- HNO3= H++ NO3- _______________________
9. Explain the basis of Ionic bonding. Give an example.
10. Explain the basis of Covalent bonding. Give an Example.
|
Problem regarding international business ethics
: Your firm has been investigating the possibility of locating facilities in an East Asian country such as Thailand, Malaysia, Taiwan, or Singapore.
|
|
Classification of costs and expenses
: Compute the physical unit flow and determine the equivalent units of production for materials and conversion - Determine the costs to be assigned to the units transferred out and in process.
|
|
Discuss any benefits associated with these worms
: Discuss any current research about the organisms you selected. What control methods are in place? What medications are available, if any, to treat the host organism. Describe the problems in the host that are associated with this particular parasit..
|
|
Essay on discrimination at work place
: Many organizations have established policies to remedy discrimination when hiring women and minorities. Discuss whether you feel that affirmative action programs, reverse discrimination, and criteria of comparable worth are appropriate forms of re..
|
|
Explain the basis of covalent bonding
: Explain the basis of Covalent bonding. Identify the following as synthesis, decomposition or exchange reaction
|
|
Essay on human resource management-mcdonalds case study
: Question : Essay on Human Resource Management in McDonalds Case Study
|
|
Discrimination against women and people belonging
: Many organizations have established policies to remedy discrimination when hiring women and minorities. Discuss whether you feel that affirmative action programs, reverse discrimination, and criteria of comparable worth are appropriate forms of re..
|
|
Legal environment of business
: Legal Environment of Business: A case study about Mary, newly joined corporate employee and her issues at work.
|
|
What circumstance might appeal to emotions become propaganda
: Under what circumstances might appeal to emotions become "propaganda"? what does the use of marketing techniques, and political consulting and campaigning techniques and/or metaphors, imply for critical reasoning, as we have discussed the latter in..
|