Reference no: EM131008609
Please provide the mechanism and major product for each of the reactions below.
1.
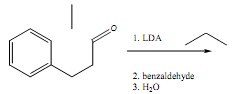
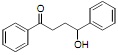
2. Propose two different retrosynthetic disconnections for the molecule below, each using a different organometallicreagent.
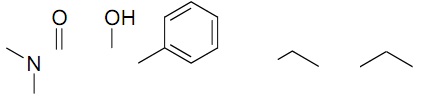
3. Label the three most acidic protons on the molecule below and rank them from lowest pKa to highest pKa. Explain yourrationale.

4. Provide the intermediate for each step in the reaction sequence, and the final product. A semicolon acts to illustrate that the reagents in a given step are not added at the same time, but sequentially, as needed. (Or, A;B = 1. A 2.B)
1. NaBH4;H2O
2. PBr3
benzaldehyde ------------>
3. Mg0
4. benzaldehyde;H2O
5. LDA;iodomethane
5. Provide a retrosynthetic disconnection strategy and mechanism for the formation of the molecule below that is essentially the dimerization of two equivalentmolecules.
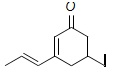
6. Draw the mechanism for the formation of the thermodynamic and kinetic products of the reaction below. Give the conditions necessary to favor the thermodynamic or kinetic product, and explain why separate products areobserved.
7. Using only styrene as a carbon source, synthesis the molecule provide below. Provide all reagents and intermediates. The first retrosynthetic disconnection should give you two molecules with equal number of carbon atoms in the"skeleton".
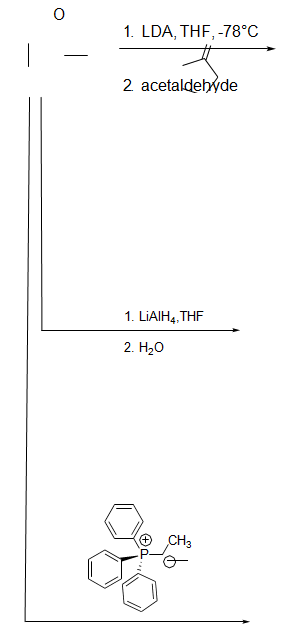
8. Provide the missing reagents and intermediates in the following multi-step synthesis. Molecule letters A and B are used for question9.
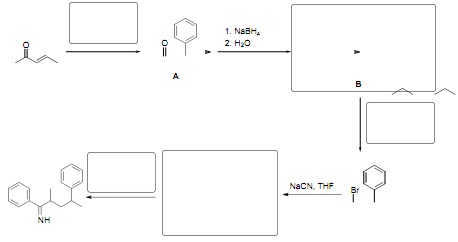
9. Assuming you carried out the second transformation in question 8 (the conversion of ketone A to molecule B) how would you use spectroscopy to confirm that all of the starting material had been consumed and that the correct product was formed? Be specific about what signals you would expect to appear anddisappear.