Reference no: EM131426498
Chemistry in silico Assignment
Coursework Assignment - In your coursework assignments you will perform simulations of dipeptide molecules of the type shown below
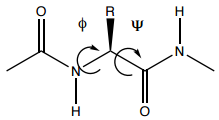
The dipeptide molecules are based on the amino acids. A given amino acid has been equipped with two minimal dipeptide bonds. The C terminus COOH is replaced by COCH3 and the N terminus NH2 is replaced by NHCH3. On the moodle page for the F14CIS course you will find a spreadsheet indicating which amino acid (given as its 3 letter code, e.g. ALA for Alanine) you have been assigned.
You will explore the potential energy surface (PES) of this molecule for the conformers associated with varying the Φ and Ψ angles.
Assignment 1: Quantum Mechanical Simulations
In this assignment you will consider how to simulate your assigned molecule quantum mechanically, studying the energy of the molecule as a function of the angles: E(Φ, Ψ). Due to steric hinderance some conformations will be energetically highly unfavourable. By calculating the optimized structures of different conformations of your molecule you will create a Ramachandran plot, showing the values of Φ and Ψ for the energetically favourable conformations you find.
The unique conformations will then be identified and ranked according to their energy. For the lowest lying chemically reasonable conformations you will calculate the harmonic vibrational frequencies and normal modes and determine their IR spectrum.
1. Quantum mechanical calculations can be highly accurate, but can also be costly. To explore the feasibility of different choices of methodology and basis set consider the formaldehyde (H2CO) and Nmethylformamide (C2H5NO) molecules. Perform geometry optimizations for each of these species and calculate the harmonic vibrational frequencies to characterize the structure you obtain as minima on the potential energy surface. Consider carefully the conformational flexibility of each molecule. Use the methods Hartree-Fock (HF), density-functional theory with the B3LYP functional, density functional theory with the ωB97XD functional and Møller-Plesset second-order perturbation theory (MP2) and the basis sets STO-3G, 3-21G, 6-31G, 6-31G∗ and 6-311G∗.
In your write up you should:
- Discuss briefly the conformational freedom of the molecules studied.
- Include a table of the energies you obtained for each method in each basis set and a table of the CPU times for each method in each basis set.
- Include plots of the energy as a function of the number of basis functions and the CPU time as a function of the number of basis functions.
- On the basis of your findings discuss which basis set oers a good compromise between accuracy and efficiency.
2. Perform a preliminary exploration of the different possible conformations of your molecule by constructing a Ramachandran plot of the allowed (Φ,Ψ) angles. This is achieved by performing a series of geometry optimization calculations at the HF/STO-3G level starting from initial geometries with Φ and Ψ varying over the range -180o to 180o in steps of 30o. A series of scripts have been constructed to help you complete this task (see the step-by-step guide, section 4.1).
In your write up you should:
- Discuss why such a small basis set and relatively crude method is used in this initial study. Hint: Consider how many calculations are required and the CPU time for each run.
- Discuss what information is contained in a Ramachandran plot.
- Include a Ramachandran plot generated from your quantum mechanical results.
- Include a table of the lowest 5 chemically reasonable conformers including their relative energy (found in the .csvfile) and the values of the (Φ,Ψ) angles. Note that someminimamay correspond to species where the atoms have re-arranged since in the starting structures only Φ and Ψ are varied, whilst the rest of the structure remains rigid. These conformations may be excluded from further consideration.
- For your lowest energy conformers include the calculated infrared spectrum.
3. Characterize the 5 lowest energy conformers on your Ramachandran plot (Φ / Ψ) for your dipeptide as minima or transition states, by performing frequency calculations. For these 5 calculations select a higher quality method and basis set combination based on your considerations in part 1.
In your write up you should:
- For your lowest energy conformer corresponding to a minimum, include the infrared spectrum and a table of the calculated frequencies and their assignments.
- Discuss how you arrived at these assignments and your choice of method / basis set.
Assignment 2: Classical Molecular Dynamics Simulations
1. Use the CHARMM molecular mechanics force field model to perform a dynamics simulation of your dipeptide molecule. See the supporting information and Workshop 3 for information on how to setup CHARMM calculations and visualize your results with the VMD program. Scripts are provided to generate Ramachandran plots and infrared spectra from your results.
In your write up you should:
- Include the Ramachandran plot and infrared spectrum from your classical simulations.
- Include a plot of the RMSD as a function of time.
- Compare and discuss the similarities / differences between your Ramachandran plots and infrared spectra from your quantum (Assignment 1) and classical simulations.
2. You should now investigate how the following parameters influence the results of your simulation (see Workshop 3 for details of how to modify the input files accordingly).
(a) the influence of the time step
(b) the influence of the length of the simulation
(c) the influence of explicit solvent
In your write up you should:
- Discuss the influence of the time step, simulation length and inclusion of solvent on your results. Which has the most significant effect?
- Present Ramachandran plots, RMSD plots, infrared spectra and simulation snapshots to evidence / support your arguments.
Attachment:- Assignment File.rar
|
Verify that given is a valid probability density function
: Verify that this is a valid probability density function.- What is the probability that the stock price increases by at least $1.00 on a randomly selected day?
|
|
What are some characteristics of a good leader
: What are some characteristics of a good leader? Please share a personal or professional experience of working with a good leader and a bad leader and what did you learn from the experience?
|
|
Transformational and charismatic leadership
: 1. What are some differences between transformational and charismatic leadership? 2. Give an example of someone who was either a transformational or charismatic leader in your life. Explain which they were and why.
|
|
Review concert report might find in a newspaper or magazine
: Each student is required to write (three) 3 concert reports during the semester. The reports should be in the form of a review one might find in a newspaper or magazine.
|
|
Describe the conformational freedom of the molecules studied
: F14CIS Chemistry in silico Assignment. In your write up you should: Discuss briefly the conformational freedom of the molecules studied. Include a table of the energies you obtained for each method in each basis set and a table of the CPU times for ..
|
|
Management as opposed to general management
: Describe project management as opposed to general management. What specific characteristics of project management make it distinct?
|
|
Analyze at least three challenges of succession planning
: Analyze at least three challenges of succession planning for your selected agency. Propose at least three components of effective implementation of succession planning.
|
|
What is the firm''s roa
: A firm has a profit margin of 15 percent on sales of $20,000,000. If the firm has debt of $7,500,000, total assets of $22,500,000, and an after-tax interest cost on total debt of 5 percent, what is the firm's ROA?
|
|
What is the mean number of hours spent driving kids
: For a family with three children, what is the probability that parents will spend less than one hour driving kids on a randomly selected day?
|