Reference no: EM131737953
Assignment -
1) Find a literature example of both:
a) a Glycosyl transferases
b) a glycosylases
That includes a mechanism. Draw and explain the step of the reaction.
2) Provide an example of a competitive reversible and an irreversible inhibitor of either class of carbohydrate enzyme.
Howe does the inhibitor work? Provide mechanistic details.
3) Provide the mechanism of a Swern oxidation.
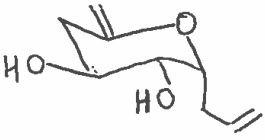
4) Form β-glucose pentaacetate:
Provide a synthesis of this sugar-like molecule.
5) a) Define chemoenzymatic synthesis.
b) Provide an example from the literature showing the starting material for the whole synthesis, the particular step using a chemoenzymatic approach, and then final product.
c) Why did they use a chemoenzymatic step?
6) Draw all 20 canonical amino acids, and label them. Be aware of Stereochemistry.
Draw 3 additional biologically common amino acids, and name them.
7) Provide protected amino acids (side chain) appropriate for a) Boc and b) Fmoc solid -phase synthesis for all 20 AAs. (Note some of the side chains of courses don't need protection). The link in the eniol should help. Note: you should draw out the full structure. Abbreviations are only helpful if you know what they mean.
8) Show deportation conditions + mechanism for the following common side chains:
- (For) (Used for Trp)
- (cyHx) (Used for Glu, Asp)
- (Xan) (Used for Asn) ← Note: why do we protect Asn but not Glu in Boc synthesis?
Use For lots
- (ODMAB) Use for Glu
- OPFP Usefull for acids
- PbF (Arg)
- Dpm (Cys)
9) Here is tripeptide
Ser-Glu-His
Provide 6 different syntheses of this system.
10) Provide a mechanism for
a) CDI
b) DCC
c) HBTCl
couplings.
11) Provide am mechanism for:
12) How can you suppless racemization during synthesis Why is it a problem? (Why do we care?)
Need to see the steps of each solution.
Attachment:- Assignment File.rar