Reference no: EM131136842
The following volumes of a standard 10.0 ppb F solution were added to four 10.00-mLaliquots of a water sample: 0.00, 1.00, 2.00, and 3.00 mL. Precisely 5.00 mL of a solution containing an excess of the strongly absorbing AI-acid alizarin garnet R complex was added to each of the four solutions, and they were each diluted to 50.0 mL. The fluorescence intensities of the four solutions were as follows:
(a) Explain the chemistry of the analytical method.
(b) Construct a plot of the data.
(c) Use the fact that the
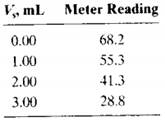
fluorescence decreases with increasing amounts of the F- standard to derive a relationship like Equation 1-3 for multiple standard additions. Use that relationship further to obtain an equation for the unknown concentration cx in terms of the slope and intercept of the standard additions plot, similar to Equcation 1-4.
(d) Use linear least squares to find the equation for the line representing the decrease in fluorescence relative to the volume of standard fluoride Vs.
(e) Calculate the standard deviation of the slope and intercept.
(f) Calculate the concentration of F- in the sample in parts per billion.
(g) Calculate the standard deviation of the result in (e).
|
Describe the vitiligo skin disease including the treatment
: Research a skin disease Vitiligo. Describe the disease/condition including the signs/symptoms and treatment. Emphasize what the coder needs to know to accurately code this disease/condition.
|
|
Complete a diagnostic battery
: Demonstrate both breadth and depth of knowledge and critical thinking appropriate to graduate-level scholarship - Introduction of the person or the character you are diagnosing
|
|
How a structured process such as six sigma methodology
: Explain in detail why data analysis skills are so important to Data Analysis Skills and Thinking. Describe how a structured process such as Six Sigma methodology (Chapter 4) can be useful to identify spending patterns and identify opportunities fo..
|
|
Illustrate the relationship between art and society
: How is art a reflection of society? Explain with at least two examples to illustrate the relationship between art and society. These two examples may be from the text or of your own selection.
|
|
Calculate the standard deviation of the slope and intercept
: Use linear least squares to find the equation for the line representing the decrease in fluorescence relative to the volume of standard fluoride Vs.
|
|
The elm street branch opens a second lane
: Scenario Three: On Friday's the Elm Street branch opens a second lane (window) during its 8 hours of operation in order to accommodate employees from a local manufacturing plant who want to deposit their paychecks. When a customer in the single line ..
|
|
Horizontal analysis of the income statement
: You will need to collect data for the latest three years for the selected companies from the financial statements and other relevant information and conduct: Horizontal analysis of the Income Statement and Cash Flow Statement and Vertical analysis ..
|
|
Calculate on the basis of mortality table
: Calculate on the basis of mortality table given below.- Net annual premium at 5% rate of interest for a five years term insurance for $1000 effective at the age of 60 years.
|
|
Should traditional sports be excluded from high school
: Should traditional sports such as Football, Volleyball and Basketball be excluded from high school PE to make room for more lifelong, individual activities like Tennis, Weight Training, Yoga, Running, Walking and overall physical conditioning? Exp..
|