Reference no: EM131044470
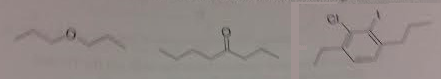
1. Provide the real name (IUPAC name) for each of the following structures.
2. Label the following as aromatic (A), anti-aromatic (AA) or non-aromatic (NA).

3. Assume you are carrying out a reduction reaction of cyclohexanone to give cyclohexanol product as shown below; describe how you will use the following techniques to monitor the reaction:

a. 1H NMR
b. IR
4. From the structure shows below, calculate the multiplicities of proton signals at positions labeled a, b and c.
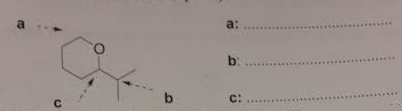
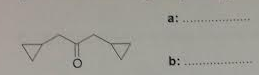
5. Write the number of signals (peaks) expected on (a) 13C NMR and (b) 1H NMR spectra of the following compound.
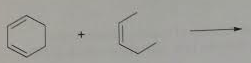
6. Provide the product (and stereochemistry) for the Diels-Alder reactions.
7. Provide the family names of the following compounds.

8. Which will be a stronger acid? Explain why it is stronger.

9. Give the fragmentation products of the compounds shown below.
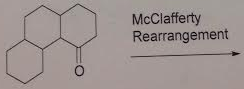
10. The following compounds al show a single line in their 13C NMR spectra. Use numbers 1-4 to label them in their expected order of increasing chemical shifts.
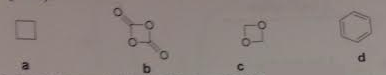
11. Provide the product in the following reactions.
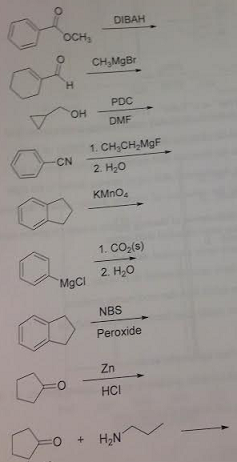
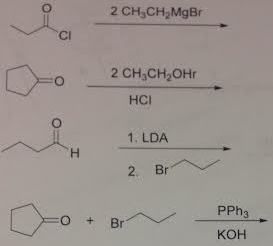
12. Provide the products for the following reactions.
13. Label each statement as "True" or a "False".
- Deshielding results in the chemical shift signal moving further down field.
- Deshielding results in the chemical shift signal moving further up field.
- Deshielding results in the chemical shift signal moving further to the left.
- Deshielding results in the chemical shift signal moving further to the right.
- Deshielding results in the reduction of electron density around the proton nuclei.
- Deshielding results in the increase of electron density around the proton nuclei,
- Homotopic protons have same chemical shift values.
- Enantiotopic protons have same chemical shift values.
- Diastereotopic protons have same chemical shift values.
- Kinetic products are less stable than thermodynamic products.
- Kinetic products are more stable than thermodynamic products.
- Epoxides are also ethers.
- Alcohols are more acidic that carboxylic acids.
- One problem with Friedel-Crafts acylation is the rearrangement of the carbocation intermediate.
- Carboxylate ions are more stable then alkoxide ions.
14. Identify the main functional group present in the spectrum below.
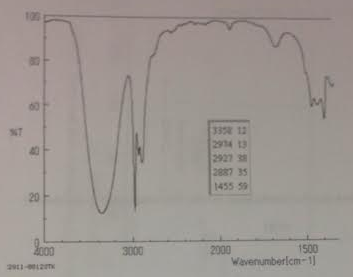
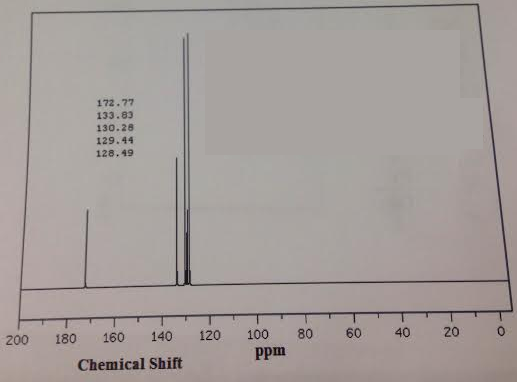
15. For the spectrum below, provide the following:
a. M+ ion
b. Base ion
c. Parent peak
d. Molecular peak
|
Advertisements-song lyrics-inventions
: For this essay-the final essay assignment of this class-you will use a variety of resources to define your generation. To create this definition, I want you to look at works of art, books, journals, advertisements, song lyrics, inventions, article..
|
|
Is there a proper role in oversight of strategic intelligenc
: Is there a proper role in oversight of strategic intelligence activities, in ensuring that the policy expressed in budget and appropriations laws is being followed, in ensuring that the will of the people and the necessity of national security is ..
|
|
Market supply curve for a product
: Which of the following statements about the market supply curve for a product is false? a) The market supply curve represents the individual supply curves of all firms which produce the product added together.
|
|
Learned from mistakes on the finance job
: Describe a typical work week for finance position? What challenges are you looking for in this finance position? What have you learned from mistakes on the finance job?
|
|
Calculate the multiplicities of proton signals
: From the structure shows below, calculate the multiplicities of proton signals at positions labeled a, b and c
|
|
Watsuji dialectic of double negation
: What does Watsuji mean when he says, "we have discovered ourselves in climate"? What does he mean when he says, "climate does not exist apart from history, nor history apart from climate?"
|
|
Determining the outstanding stock
: Assume the following for aol corp for 2012. cash flow from assets is minus $660; interest expense = $460; dividends paid = $1280 and long term debt is unchanged from 2011. What did aol corp do with regard to its outstanding stock during 2012
|
|
Total long-term debt and total liabilities
: a. What were Wallace's total long-term debt and total liabilities in 2013? b. How much new long-term debt financing will be needed in 2014? (Hint: AFN - New stock = New long-term debt)
|
|
How first account of creation related to babylonian creation
: According to Coogan, how is the first account of creation related to the Babylonian creation narrative Enuma Elish? What purposes do the changes serve?
|