Reference no: EM132786336 , Length: 99 words
Question 1: A 0.517 g sample of a metal, M, reacts completely with sulfuric acid according to
M(s)+H2SO4(aq)?MSO4(aq)+H2(g)
A volume of 235 mL of hydrogen is collected over water; the water level in the collecting vessel is the same as the outside level. Atmospheric pressure is 756.0 Torr, and the temperature is 25 °C. The vapor pressure of water at 25 °C is 23.8 Torr. Calculate the molar mass of the metal.
Question 2: Is the following reaction an oxidation, reduction, or neither?

oxidation
reduction
neither
Question 3: What is the structure proposed from the below data?
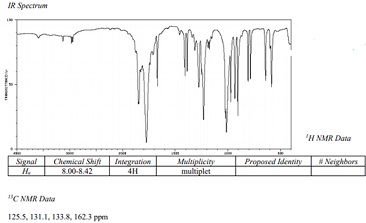
Molecular Formula: C8H4O3
Question 4: Which of the fowling coordination compounds in color?
A) [Co(NO2)6)]3- B)[CO(NH3)6]3+ C) [Ag(NH3)2]+ D) [Ti(H2O6)]3+
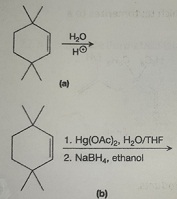
Question 5: Draw the mechanism for the reaction below:
Question 6: Calculate the molarity of each of the following solutions.
0.500 mole of urea (CH4N2O) in 2.50 × 102 mL of solution
Question 7: Write out the correct answer to the math problem below, paying close attention to significant figures. Write as a normal decimal number. Do not use scientific notation.
(0.0590x8.105x102/1.8x10-3) =
Question 8: Name the following organic compounds using the IUPAC system