Reference no: EM131261830
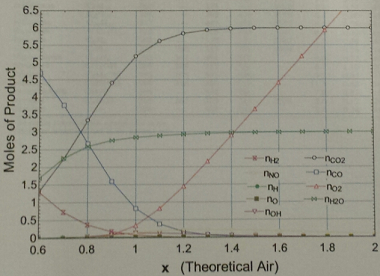
Q1. Below is a graph showing the moles of product produced when burning benzene (C6H6) in air as a function of % theoretical air. This graph was generated based on the equilibrium reactions discussed. Please answer the following questions related to the graph:
a) Why are the moles of CO and O2 at 100% theoretical air (x = 1) above zero? Recall that 100% theoretical air is the stoichiometric condition.
b) Why do the moles of CO go to zero (approximately) at 160% theoretical air (x = 1.6)? If appropriate explain the difference with part a.
c) Why are the moles of CO and 112 larger at 80% theoretical air (x = 0.8) compared to 100% theoretical air?
Q2. If liquid benzene spills on a lab table that has an open flame, will it catch on fire? The table is at -30°C and cools the benzene to this temperature. Quantitatively explain your answer. Also, what does your mathematical model physically represent? Use the following information to answer the question:
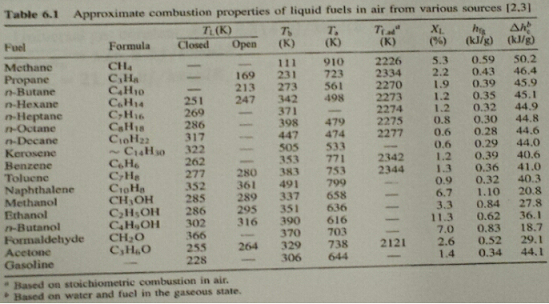
Q3. A stoichiontetric mixture of benzene and air at T1 = 300 K. P1 = 100 kPa, and V1 = 5 liters (0,005 m3) is compressed in a piston-cylinder assembly in a polytropic process where n = 1.2. If the final pressure is 5000 kPa, will the mixture autoignite? The autoignition temperature for benzene is 771 K. Also, calculate the work in kJ and the heat transfer in kJ required to raise the mixture pressure to 5000 kPa. Use the information below in answering the questions:
Universal gas constant: RU = 8.314 kJ/kmolK
(Cv,C_6H_6)- = 126.6 kJ /kmolK,
(Cv,air) = 21.27 kJ/kmolK,
Q4. Benzene (C6H4) is burned in air. You may assume the mass fraction of oxygen in air is 0.23 and the rest of the oxidizer stream is nitrogen. Answer the following:
a) Calculate the mixture fraction at stoichiometric condition.
b) Calculate the mixture fraction of the products at the stoichiometric mixture fraction.
c) Plot the mass fractions of the reactants and products versus mixture fraction.
d) What are the mass fractions of products and reactants at a mixture fraction of 0.5?