Reference no: EM131249385
Consider a colloidal suspension of latex particles confined between two plates. The plates are charged, so there is an electric potential difference V between them (as shown in the figure below). The number of particles is N and the temperature is T. The system is isolated from the environment and gravitational effects are negligible.
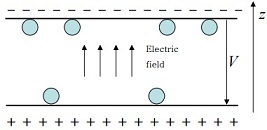
Now each particle carries a positive charge +q, so particles are more likely to be near the plate at the top in the figure, which is negatively charged, than the bottom one, which is positively charged. The system is very dilute, so particles are independent of each other and the electrostatic interaction between particles can be neglected. For simplicity, assume that in the z direction each particle may only be at one of the following two locations: either attached to the top plate, or attached to the bottom one, as shown in the figure.
(i) Calculate the fraction of particles attached to the top plate. Sketch this as a function of: (a) T, (b) V. Discuss the physical meaning of your results.
Hint for (i): The total energy of the system, E, depends on how the particles are distributed between the two plates. Find out the number of configurations associated with a certain E and express the entropy of the system, S, as a function of E, V, and N. Then use T = ∂E/∂S|V,N and solve for E.
(ii) Consider the case at room temperature (300±K) and q = 10 e, where e is the magnitude of the charge carried by an electron (1.6µ10-19 C). (This is due to the ionization of 10 charged groups chemically attached to the particle surface.) How large does V need to be so that the particles have a significant preference to reside at the top plate? (In reality, there is salt in the solution, and the salt ions interact with the particles in a complex way and partially neutralize them. So q here should be regarded as the effective charge carried by a particle after considering these effects.)
|
Impact in the colonial era
: Choose a major theme that had the most impact in the colonial era. Analyze it in terms of its strengths and weaknesses. How did it reflect the needs of society at that time? Is there a comparable example that you can identify in today''s society?
|
|
Example of indenture contracts
: Search the Internet for additional examples of indenture contracts like those found in the text. Post the URL you find and explain why you found it useful and informative in understanding early American welfare policies.
|
|
Treatment of forensic populations - final project
: Identify which scenario you selected to use for your Final Project and Explain the offender category (ies) that concurs with the scenario client and discern the treatment considerations inherent in this category (ies).
|
|
Elasticity of the overall product market
: Why would the price elasticity of demand for an individual firm's product be greater than the elasticity of the overall product market?
|
|
Calculate the fraction of particle attached to the top plate
: Calculate the fraction of particles attached to the top plate. Sketch this as a function of: (a) T, (b) V. Discuss the physical meaning of your results.
|
|
Calculate the price of a prepaid forward contract
: Suppose a company's $50 stock pays an 8% continuous dividend and the continuously compounded risk-free rate is 6%. Calculate the following: the price of a prepaid forward contract that expires 1 year from now
|
|
Marginal rate of technical substitution
: Graph an isoquant for output of q=12. Label this line 'isoquant'. What is the marginal product of B? What is the marginal rate of technical substitution at each point on an isoquant?
|
|
Should transportation security regulations be changed
: Should Transportation Security (TSA) regulations be changed? - Should regulations regarding the use of cell phones while driving be standardized?
|
|
Favor of reducing taxes on repatriated earnings
: Some have argued in favor of reducing taxes on repatriated earnings that companies operating in the United States have made in other countries. Such a tax break could lead to a sharp increase in the amount of repatriated earnings and raise tax rev..
|