Reference no: EM131216375
Q1. Benzene (MW = 78 g/mol) is being released by accident from a submerged source in the ocean at a constant flow rate of V· = 0.1 m3/s as shown below. Since the density of the benzene (ρ = 876 kg/m3) is less than that of water and since the benzene is only sparingly soluble in water, a slick of benzene will form on the water surface. Once the slick is formed, it will have a tendency to spread as more benzene is added from the source but also a tendency to shrink as the benzene both evaporates to the air and dissolves in the water. Eventually, it will reach a constant size at steady state.
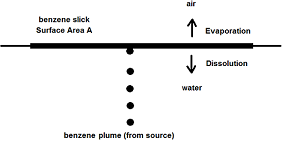
At steady state, a mole balance on the benzene in the slick is:
rate of benzene to slick from the source = rate of dissolution + rate of evaporation
The mass transfer coefficient hm for dissolution of benzene into the water is 9.2 cm/hr and is 1240 cm/hr for the evaporation of benzene into air. The concentration of benzene in the water at the slick-water interface is dictated by its solubility in water and is 22.8 mol/m3. The concentration of benzene in the air at the slick-air interface is dictated by its vapor pressure and is 5.1 mol/m3. Estimate the area (in m2) of the slick at steady state. Assuming it was circular, what would its diameter (m) be?
Q2. The purpose of this exercise is to determine the experimental value of the diffusion coefficient of sodium chloride through a cellulose membrane. Refer to Figure 1 for the experimental set-up. Initially, V=100 cm3 of sodium chloride aqueous solution with a salt concentration of 5 mol/L is placed in the small cup. A cellulose membrane forms the base of the cup and sodium chloride begins to diffuse through it to the volume V2 below. Volume V2 is pure water and is continuously replenished so it may be assumed that the salt concentration at any time is effectively zero. As time goes on, the concentration of NaCl in V decreases. This is an unsteady-state process.
During the experiment, the electrical conductivity of the solution in V is measured and a calibration curve of conductivity versus NaCl concentration is used to prepare data of NaCl concentration in V as a function of time. These data are in an EXCEL sheet that you can find in the assignments folder of the course canvas site. You will use these data to find the effective diffusion coefficient Deff of NaCl through the membrane.
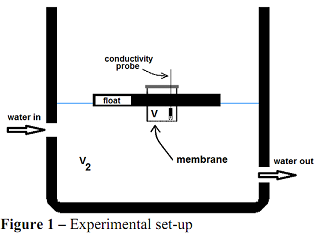
We will neglect convective resistances on both sides of the membrane and consider the only resistance to mass transfer to be in the membrane itself. This results in a mass transfer model:
ln(C/C0) = -bt (1)
where C is the concentration of salt in V at any time and C0 is the initial concentration of salt in V. The symbol b is given by:
b = [DeffA/Vδ] (2)
where A is the cross sectional area of the circular membrane and δ is its thickness. The diameter and thickness of the membrane are 9.0 cm and 2.25 μm respectively.
(a) Using EXCEL and the provided concentration data, determine the value of b in equation (1) that best represents the data. Make sure you specify its units. Submit your EXCEL sheet as a hard copy with the rest of the assignment.
Also, make a graph of concentration versus time in which the experimental values are represented as discrete points and the calculated values as a continuous curve.
(b) Use equation (2) and your result from (a) to calculate the effective diffusivity of salt in the membrane. Express your answer in cm2/hr.
|
Find the pdf of the time
: Find the mean time that the toll collector will require to serve cars that arrive in a one-minute interval.- Find the PDF of the time that the toll collector will require to serve cars that arrive in a one-minute interval.
|
|
Concerning the financial statements prepared
: On January 1, 19xl, Bennett Company paid $1,000,000 to acquire all of the outstanding stock of Frazier Company. At that date the balance sheet of Frazier Company showed total assets of $1,500,000, total liabilities of $500,000, common stock of $700,0..
|
|
Find the probability generating function of s
: A random sum of discrete IID random variables.- Find the probability-generating function of S assuming that is independent of the XK.
|
|
What is your understanding of effective communication
: How do you see the four-step process working in a real situation with an angry client? What else might you need to be aware of when dealing with an angry client and why? Give examples.
|
|
Calculate the effective diffusivity of salt in the membrane
: Use equation (2) and your result from (a) to calculate the effective diffusivity of salt in the membrane. Express your answer in cm2/hr
|
|
What is the pgf of the gamblers total winnings
: What is the PGF of the gambler's total winnings after playing for an hour?- What is the probability that the gambler has not lost any money after an hour.
|
|
Different depreciation methods-annual depreciation expense
: Boscan Corporation purchased machinery on January 1, 2014, at a cost of $270,000. The estimated useful life of the machinery is 4 years, with an estimated salvage value at the end of that period of $26,000. The company is considering different deprec..
|
|
Do you think increased media attention on bullying warranted
: In your view, what are the best ways for professionals who work with school-age children to address bullying (e.g., zero tolerance policies, anti-bullying contracts, role play, lessons, etc.)? Identify and explain at least two.
|
|
Beyond the split-off point will cause profits
: Duiker Corporation has a joint process that produces three product: A, S and B. Each product may be sold at split-off or processed further and then sold. Joint-processing costs for the year amount to $30,000. Processing Product S beyond the split-off..
|