Reference no: EM13968776
Mass, Volume and Density Lab
Objectives:
- to measure different volumes of liquid using significant figures
- to measure the mass of objects in the appropriate number of significant figures with a balance/scale.
- to use careful measurements to determine the density of a substance.
- to observe how substances of different densities interact.
Procedure: There is a separate document for you to download and enter your data (the datasheet). When you are done, submit the document in the Lab Dropbox. Please see the accepted formats (.pdf, .docx preferred but .rtf or .doc ok too.)
Part I: Volume of water.
1. Fill the graduated cylinder (note the difference between it & the beaker) about ½ full of water.
2. Read the volume with the appropriate number of significant figures and include the correct unit. The question is how do you know the correct number of significant figures to report? You have to take a look at your measuring device. Checking to see what the units are (L, mL or uL) and how many divisions are between the marked values. Note: For our graduated cylinder the units are ml and the marked values are 10, 20, .....50 ml. Between each of the marked values are 9 divisions, which means that each division is 1 ml. However, a liquid measure can be between each of 1 ml increments and you can estimate where it is. Thus you can read to the 0.1 place or to the tenths place. Because significant figures include every digit you know for sure plus one digit that is estimated, your record of the measurement of the volume of water should include the 0.1 place, such as 24.5 ml or 25.2 ml. What if the water level falls right on a division? Simple- the tenths place would have a value of 0, i.e., 24.0. To read the water level, look at the boundary between the water and the air.
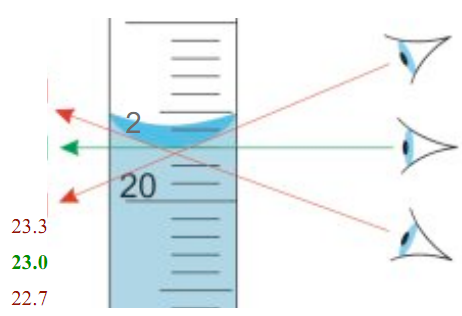
The boundary is known as the meniscus. When you look at the water through the side of the cylinder, you may notice that some crept up the edges. You need to read the value at the most horizontal, lowest portion of the meniscus, which is towards the center of the cylinder. 23.0 mL is the correct reading in the picture to the right because the bottom of the meniscus is right on 23.
Part II: Mass of solid objects
1. Find a stable place to put your balance. Turn it on and press the "tare" or "zero" button so that it reads "0.0g".
2. Place the empty beaker on the balance. Record value of the empty beaker, remembering to use the appropriate number of significant figures and include the correct unit.
3. Add a small object to the beaker and record the new mass in "beaker + object".
4. Subtract the two values to obtain the mass of the object alone. Watch for the significant figure rule on addition/subtraction here!
5. Take the object out of the beaker but leave the beaker on the balance. Press "Tare" or "Zero" (depending upon which type of balance you have.) Then place the object in the beaker and record it's mass.
Part III: Determining the density of solids
If we had a regular shaped object such as a rectangular eraser or a cube, density would be very straight forward: 1) measure L x W x H for the volume, 2) measure the mass with the balance, 3) calculate density. It is harder to directly measure the volume of irregular shaped objects, so we have to do it by volume displacement. Think about drawing a nice hot bath almost to the top of your tub. As you get in the tub, all ready for the relaxation that the bath will bring, the water spills out of the tub!! The volume that your body takes up has pushed an equivalent volume of water out of the way to make room for you!
1. Find the mass of the solid, as above.
2. Fill the cylinder about ½ way with water. Record the starting volume of the water.
3. Select one of your small objects, the one that is more than 5 grams, measure and record its mass.
4. Carefully place the object in the graduated cylinder (you don't want water to splash up the sides or out of the cylinder!). Record the new volume water.
5. Calculate the density of the object and record the result. Watch for the significant figure rule on multiplication/division here!
6. Repeat the above steps with your similar objects that are less than 5 g. Put all of them on the balance and then all of them in the graduated cylinder for mass and volume respectively.
7. What if your object did not sink in the water? That just means the density of the object is less than the density of the water - so pick a different object.
Part IV: Determining the density of liquids
1. Determine the mass and precise volume (i.e. 5.0 or 5.5 etc.) of approximately 5 ml of rubbing alcohol.
Hint: To do this, put the graduated cylinder on the balance and then press tare/zero. Pour about 5 mL of rubbing alcohol in. Then read the mass and volume (remember, the balance has already subtracted out the mass of the graduated cylinder.)
2. Determine the mass and precise volume (i.e. 10.0 or 10.5 etc.) of approximately 10 ml of rubbing alcohol.
Hint: Just add to what you did in step 1 (make sure you haven't pressed tare/zero again though.)
3. Determine the mass and precise volume (i.e. 15.0 or 15.5 etc.) of approximately 15 ml of rubbing alcohol.
4. Calculate the density of each of the solutions you measured.
Part V: Layering liquids
1. Take a tall clear glass or clear plastic cup (12 oz works great, but if you have only 8 oz plastic cup, just cut back on the volumes of the liquids a little bit).
2. Pour the syrup or honey into the middle of the glass. Be careful not to get any one the sides of the glass!. Pour enough syrup in to fill the glass about 1/6 of the way.
3. Tip the glass slightly and pour an equal amount of the dishwashing liquid slowly down the side of the glass. Observe whether the dishwashing liquid floats or sinks.
4. Next mix a few drops of food coloring with water in one the mixing cups.
5. In another mixing cup add a few drops of a different color to the rubbing alcohol. DO THE NEXT STEPS VERY SLOWLY.
6. Tip the glass slightly and pour the colored water slowly down the side of the glass. This is to prevent mixing of the layers due to agitation.
7. Then pour the vegetable oil slowly on top of the water.
8. Lastly, add the colored rubbing alcohol.
Attachment:- Density-Datasheet.rtf