Reference no: EM132657953
CHT353 Drug Targets - Cardiff University
Enzyme Inhibition
Question 1. The reaction of carbon dioxide with water (shown below) is catalyzed in the eye by the enzyme carbonic anhydrase II (HCA II). This is an important reaction because it is involved in maintaining the shape of the eyeball. Unfortunately, too much HCA II activity can lead to high pressures in the eye and glaucoma.
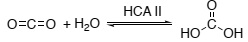
Because water is in large excess, the enzyme-catalyzed rate of carbonic acid (H2CO3) as a function of [CO2] follows Michaelis-Menten steady-state kinetics. Write down the Michaelis- Menten equation for HCA II and hence derive the following expression:
vo = Vmax - KM.vo/[CO2]
Where vo is the initial rate, Vmax is the maximum rate, and KM is the CO2 concentration at which vo = 0.5Vmax.
Question 2. Calculate the minimum time taken for CO2 to react with water in the presence of HCA II given that 10-6 moles of the enzyme produces 0.6 moles of carbonic acid (H2CO3) per second (Vmax).
Question 3. Competitive inhibitors of HCA II, such as Trusopt (shown below), are used to reduce high internal pressures of CO2 in the eye. Draw a plot showing how vo versus vo/[S] changes for the HCA II-catalysed production of H2CO3 in the presence of increasing concentrations of Trusopt.

Hint: Use a suitably modified form of the expression you derived above.
Question 4. Briefly explain why uncompetitive inhibitors are more effective at stopping enzyme-catalyzed reactions in vivo (in a living cell) than competitive inhibitors, and hence explain why most drugs are deliberately chosen to be competitive inhibitors.
Ligand Binding
Question 5. When water molecules surround methane (CH4) the enthalpy of the system decreases. This is due to the formation of dispersion interactions and strong hydrogen bonds between water molecules on the surface of the methane molecule. Yet methane is almost completely insoluble in water, the free energy change (ΔGsol) associated with moving methane from the gas phase into aqueous solution being ΔGsol = +26.4 kJ/mol. Briefly explain why ΔGsol is so unfavorable.

Question 6. Somewhat counter-intuitively, most of the interaction energy needed to form molecular complexes in water is provided by dispersion interactions between hydrophobic groups located on separate molecules. Provide evidence to support this assertion by calculating the free energy for the dimerization of two CH4 molecules in water (ΔGdim) given the following information:

Question 7. Again counter-intuitively, the contribution of hydrogen bonds to ligand binding affinity is also less than might be expected based on their energies in the gas-phase. For example, studies of intermediate binding to the enzyme tyrosyl-tRNA synthetase showed that removing a hydrogen bond to an uncharged group on the substrate by changing Tyr-34 to a phenylalanine residue (see figure) decreased the binding energy by only 2 kJ/mol. This is surprising given that similar hydrogen bonds in the gas-phase have an energy of approximately 20 kJ/mol. Briefly explain this experimental observation.